P475 Association between disease duration and degree of endoscopic improvement in patients with ulcerative colitis treated with tofacitinib
W.J. Sandborn1, J.P. Gisbert2, I. Modesto3, C. Su4, D. Quirk4, R. Mundayat3, X. Guo4, M. Chiorean5, W. Reinisch6
1Division of Gastroenterology- University of California, San Diego, La Jolla- California, USA, 2Gastroenterology Unit, Hospital Universitario de La Princesa- IIS-IP and CIBERehd, Madrid, Spain, 3Pfizer Inc., New York, New York, USA, 4Pfizer Inc., Collegeville, Pennsylvania, USA, 5Virginia Mason Medical Center, Seattle, Washington, USA, 6Medical University of Vienna, Vienna, Austria, Austria
Background
Tofacitinib is an oral, small-molecule JAK inhibitor for the treatment of ulcerative colitis (UC). Efficacy and safety of tofacitinib in patients with UC were evaluated in two Phase 3 induction studies (OCTAVE Induction 1 and 2; NCT01465763, NCT01458951), a 52-week, Phase 3 maintenance study (OCTAVE Sustain, NCT01458574), and an ongoing, open-label, long-term extension study (NCT01470612) [1,2]. Here, we evaluate the association between disease duration and the likelihood of achieving an endoscopic subscore of either 0 or 1 in OCTAVE Induction 1 and 2 and OCTAVE Sustain.
Methods
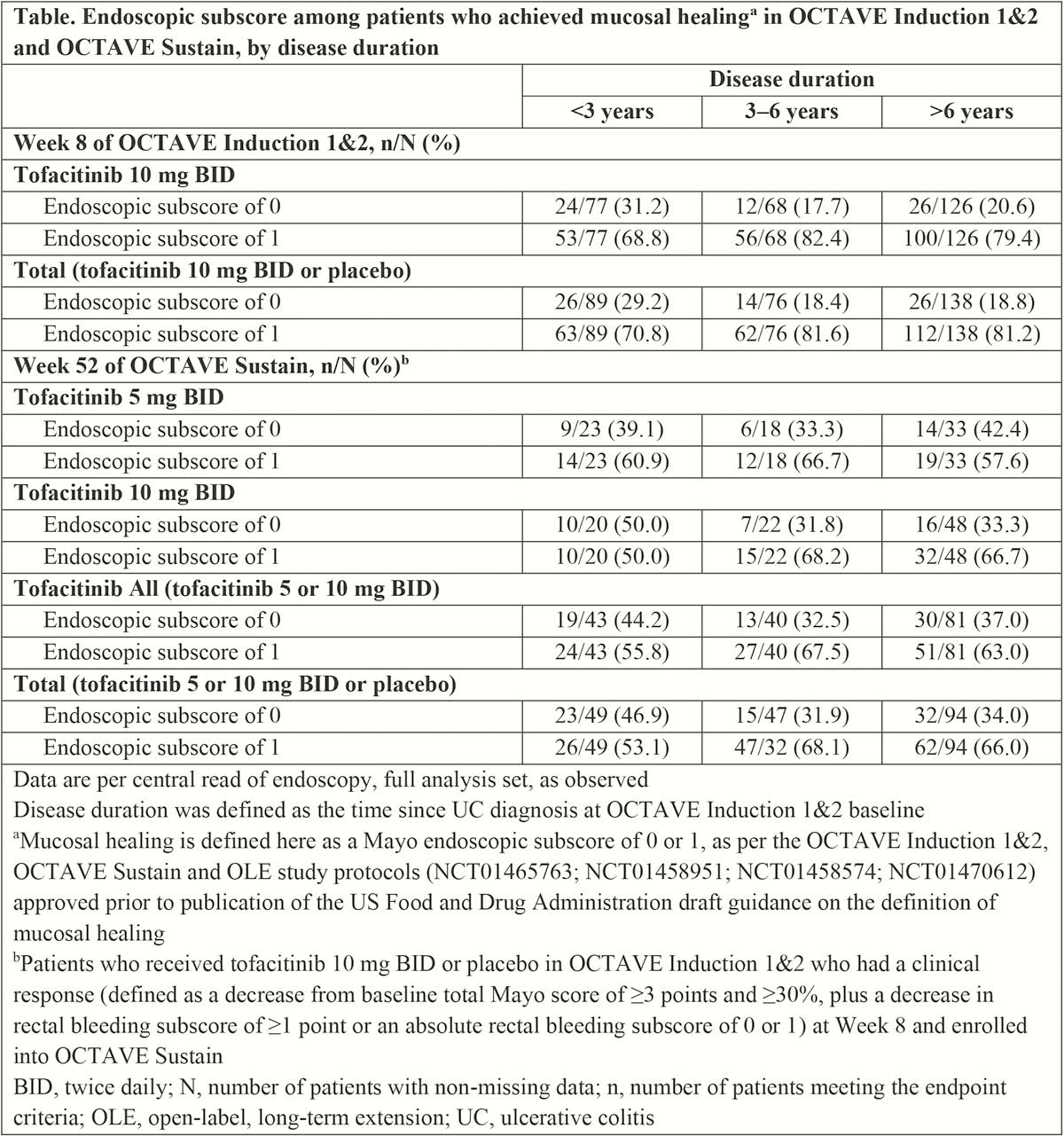
The degree of mucosal healing (Mayo endoscopic subscore of 0 or 1) achieved at Week 8 of OCTAVE Induction 1 and 2 (tofacitinib 10 mg twice daily [BID] or placebo) and at Week 52 of OCTAVE Sustain (responders from OCTAVE Induction 1 and 2; tofacitinib 5 or 10 mg BID or placebo) was evaluated in relation to UC disease duration at OCTAVE Induction 1 and 2 baseline (ie prior to study treatment). The association between disease duration category (<3, 3–6 and >6 years) and endoscopic subscore (centrally read) was assessed using the Cochran–Mantel–Haenszel chi-square test. Patients who did not achieve mucosal healing (as observed) were excluded.
Results
A total of 303/1139 (26.6%) patients achieved mucosal healing at Week 8 of OCTAVE Induction 1 and 2, and 190/593 (32.0%) at Week 52 of OCTAVE Sustain. Of patients who achieved mucosal healing at Week 8 of OCTAVE Induction 1 and 2 (with tofacitinib or placebo), 89 (29.4%), 76 (25.1%) and 138 (45.5%) had baseline disease durations of <3 years, 3–6 years and >6 years, respectively. Overall (among patients who achieved mucosal healing, treated with tofacitinib or placebo), no statistically significant association between disease duration and endoscopic subscore was observed, either at Week 8 of OCTAVE Induction 1 and 2 or at Week 52 of OCTAVE Sustain (chi-squared
Conclusion
Among patients with endoscopic improvement after induction and maintenance therapy with tofacitinib, rates of complete mucosal healing were numerically higher among those with UC for <3 years before starting treatment than among those with a longer disease duration. These analyses are post hoc and limited by the small sample size.
Sandborn WJ et al. N Engl J Med 2017;376:1723–36
Lichtenstein GR et al. United European Gastroenterol J 2019;7:Abstract OP213


