P478 Platelet-related indicators for predicting vedolizumab response in patients with Ulcerative Colitis
Jiao, J.(1)*;Liu, W.(1);Wang, B.(1);Cao, H.(1);
(1)Tianjin Medical University General Hospital, Department of gastroenterology and hepatology, Tianjin, China;
Background
Ulcerative Colitis (UC) combine both inflammation and coagulation in their pathogenesis and clinical manifestations. Although platelets (PLT) are well known for their role in hemostasis, there are a rising number of studies supporting their considerable role as inflammatory amplifiers in chronic inflammatory conditions. However, whether PLT are related to the efficacy of biologic agents is unknown. This study aimed to investigate whether platelet ratio before and after induction therapy can be predictive biomarkers for the therapeutic outcomes of vedolizumab therapy in UC.
Methods
Patients with moderate-to-severe UC receiving vedolizumab in our hospital between November 2020 and November 2022 were retrospectively evaluated. Patients with concomitant corticosteroid treatment≥20 mg, immunomodulators, or other conditions that could modify the platelet count were excluded. The primary endpoint was clinical response at the end of the induction period (at 14 weeks). We analyzed the association between platelet count, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), C-reactive protein (CRP), fecal calprotectin, and the clinical efficacy of vedolizumab. The above indicators between the response and nonresponse groups were compared via a univariate analysis. The independent risk factors of nonresponse were identified via a multivariate analysis. 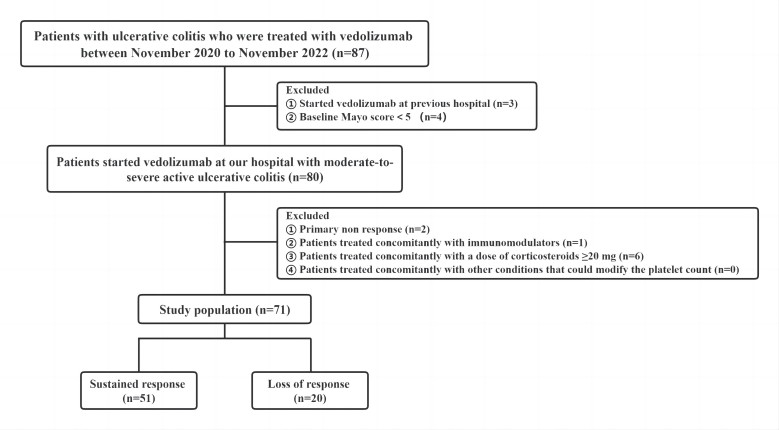
Results
Seventy-one patients with moderate-to-severe active UC started treatment with vedolizumab and 20 patients (28.2%) experienced loss of response (LOR) after induction therapy. The platelet count, albumin, CRP, NLR, PLR, MLR, and Particul Mayo score between the LOR and response groups significantly differed. The Platelet ratio before and after induction therapy cut-off value of 1.077 was predictive of LOR, using receiver operating characteristic analysis (sensitivity:78.0%, specificity:81.8%). A univariate analysis revealed a significant relationship between LOR and the platelet ratio (P<0.05). Multivariate analysis indicated the platelet ratio as an independent prognostic factor for LOR (OR=11.219, 95% CI:3.316-37.959, P<0.05).
Conclusion
Platelet ratio before and after induction therapy is a useful prognostic marker in patients with moderate-to-severe active UC treated with vedolizumab, and may contribute to appropriate use of vedolizumab LOR. A large sample from multiple centers is needed to validate the association between platelet-related measures and the rate of vedolizumab response.


