P478 The role of proactive measurement of adalimumab trough levels and antibodies to adalimumab in Greek patients with Inflammatory Bowel Disease
Orfanoudaki, E.(1);Gazouli, M.(2);Theodoraki, E.(1);Foteinogiannopoulou, K.(1);Andreou, N.P.(2);Koutroubakis, I.E.(1);
(1)University Hospital of Heraklion- Medical School- University of Crete, Gastroenterology, Heraklion Crete, Greece;(2)Medical School- National and Kapodistrian University of Athens- Greece, Department of Basic Medical Sciences- Laboratory of Biology, Athens, Greece;
Background
Therapeutic drug monitoring (TDM) of adalimumab trough levels (ADA-TLs) and antibodies to adalimumab (ADA-Abs) in patients with inflammatory bowel disease (IBD) is usually performed in a reactive setting when the disease flares. There is not enough evidence in favor of proactive TDM in these patients.
Methods
We aimed to evaluate the role of proactive measurement of ADA-TLs and ADA-Abs in IBD patients under treatment with adalimumab. Consecutive IBD patients on maintenance treatment with ADA, were included. ADA-TLs and Abs were measured using ELISA (Immunoguide, Aybayteche, Turkey) on serum samples drawn before ADA administration. At the same time serum biomarkers [Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), white blood cells (WBC), hemoglobin (Hgb), platelets (PLT)] as well as home-based fecal calprotectin (FC) ±3 months from blood sampling were assessed. After the measurements patients were followed and treatment changes made were recorded.
Results
A total of 52 patients receiving ADA maintenance therapy [48 CD, 4 UC, 31 males, mean age (±SD) 42.1±12.6 years, 8 on combination therapy with immunomodulators (IMMs), 23 under intensified dose (either 40mg/w or 80mg/2w)] were included. Median (IQR) time since ADA initiation was 31 (17.5-38.5) months and median value (IQR) of serum ADA-TLs was 8.24 μg/ml (3.32-10.02). Five out 52 (9.6%) were positive for ATIs (>3 AU/ml). Patients with positive Abs had median (IQR) ADA-TLs 0.41 μg/ml (0.10-0.71) statistically lower compared to those with negative Abs [8.86μg/ml (5.6-10.0) (p=0.0004)]. The correlations of ADA-TLs with disease characteristics, treatment and biomarkers in IBD patients are presented in Table 1. Elevated CRP was negatively associated with ADA-TLs. No other significant correlations were observed except a trend towards significance for body mass index (BMI) and ADA treatment duration (both p=0.09). After a median (IQR) follow up of 15 (11.5-16) months treatment optimization became necessary in 4 patients (8%) [adalimumab dose intensification in 2, change to a clinical trial medication in 1 and 1 underwent surgery] who initially had significantly lower ADA-TLs than the rest patients [median (IQR) ADA-TLs 0.51μg/ml (0.26-5.09-10.04) vs 8.62 (4.48-10.04); p=0.0362].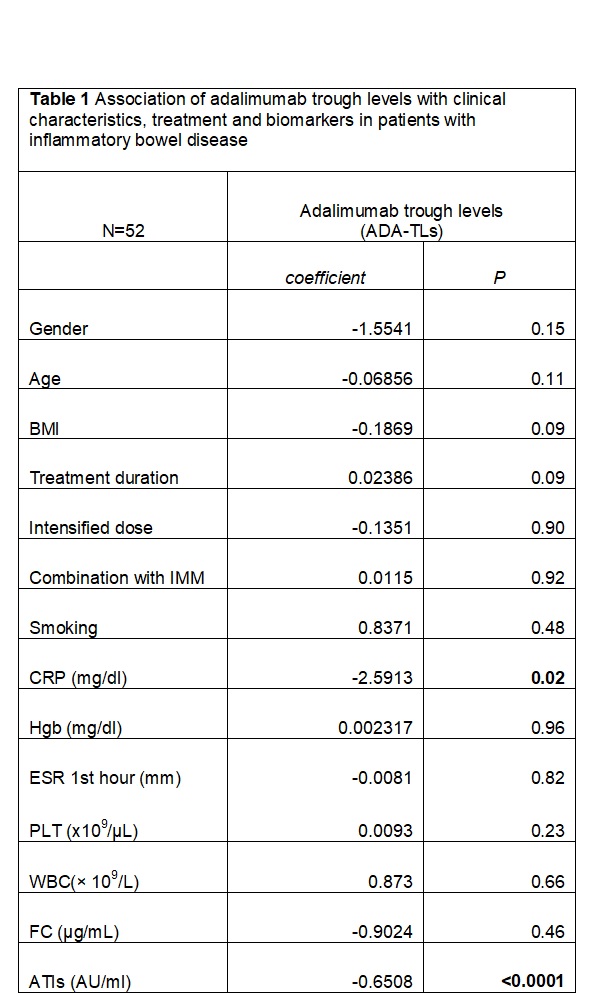
Conclusion
Proactive measurement of ADA-TLs and Abs in patients under maintenance treatment with adalimumab may be worth using along with other parameters in the management of IBD patients. Additional long term research in larger populations focused on the optimal use of proactive TDM is needed.


