P483 Vedolizumab serum drug levels are not associated with clinical outcomes or markers of disease activity in inflammatory bowel disease
S. Shields1, J.P. Seenan1, E. Nowell1, A. Dunlop2, P. Galloway2, J. Macdonald1
1Gastroenterology, Queen Elizabeth University Hospital, Glasgow, UK, 2Biochemistry, Queen Elizabeth University Hospital, Glasgow, UK
Background
Treatment options in Inflammatory Bowel Disease (IBD) include anti-TNFα and anti-integrin biologics. Therapeutic drug monitoring (TDM) is used to support clinical decision making and improve treatment outcomes with anti-TNFα drugs infliximab and adalimumab.1,2 It is unclear if TDM can offer similar benefits with vedolizumab (VDZ) and there are no clinical guidelines for using TDM with VDZ3. The aim of this study was to identify if drug levels (DL) are associated with clinical outcomes and markers of disease activity in patients treated with VDZ.
Methods
All VDZ DLs performed since introduction of testing at our unit in December 2018 were retrospectively identified. Target DLs of >30 μg/ml during induction (≤14 weeks since treatment initiation (TI)) and >10 μg/ml in maintenance (>14 weeks after TI) were agreed based on available published data. Dose information, timing of DL, lab results and disease activity scores were obtained from electronic patient records and paired with the DL dose. Calprotectin was included if recorded within 3 months of DL. Patients were classified as in remission or mild, moderate or severe active disease according to partial Mayo Score for ulcerative colitis and Harvey–Bradshaw Index for Crohn’s disease. Sub-analysis was undertaken according to timing of testing in relation to TI.
Results
Sixty pre-dose trough VDZ levels were identified from 41 patients. Median VDZ level was 25.7 μg/ml (<3.5–>70). Median time from TI to first VDL DL was 36 weeks (4.6–184). No relationship was identified between VDZ levels and biochemical markers of disease (Figure 1). Fourteen (23.3%) TDM were performed during induction. 6/14(42.8%) had subtherapeutic DLs; 3 in remission, 1 mild, 2 severe disease. The remaining 8/14(57.1%) had therapeutic DL; 3 mild, 3 moderate, 1 severe disease, 1 unknown. Forty-six (76.7%) TDM were performed during maintenance. Five out of 46(10.9%) had subtherapeutic DLs; 3 remission, 1 moderate disease, 1 unknown. The remaining 41/46(89.1%) had therapeutic DLs; 16 remission, 11 mild, 10 moderate, 3 severe disease, 1 unknown. For both sub-analysis groups, no relationship between disease state and drug level was observed.
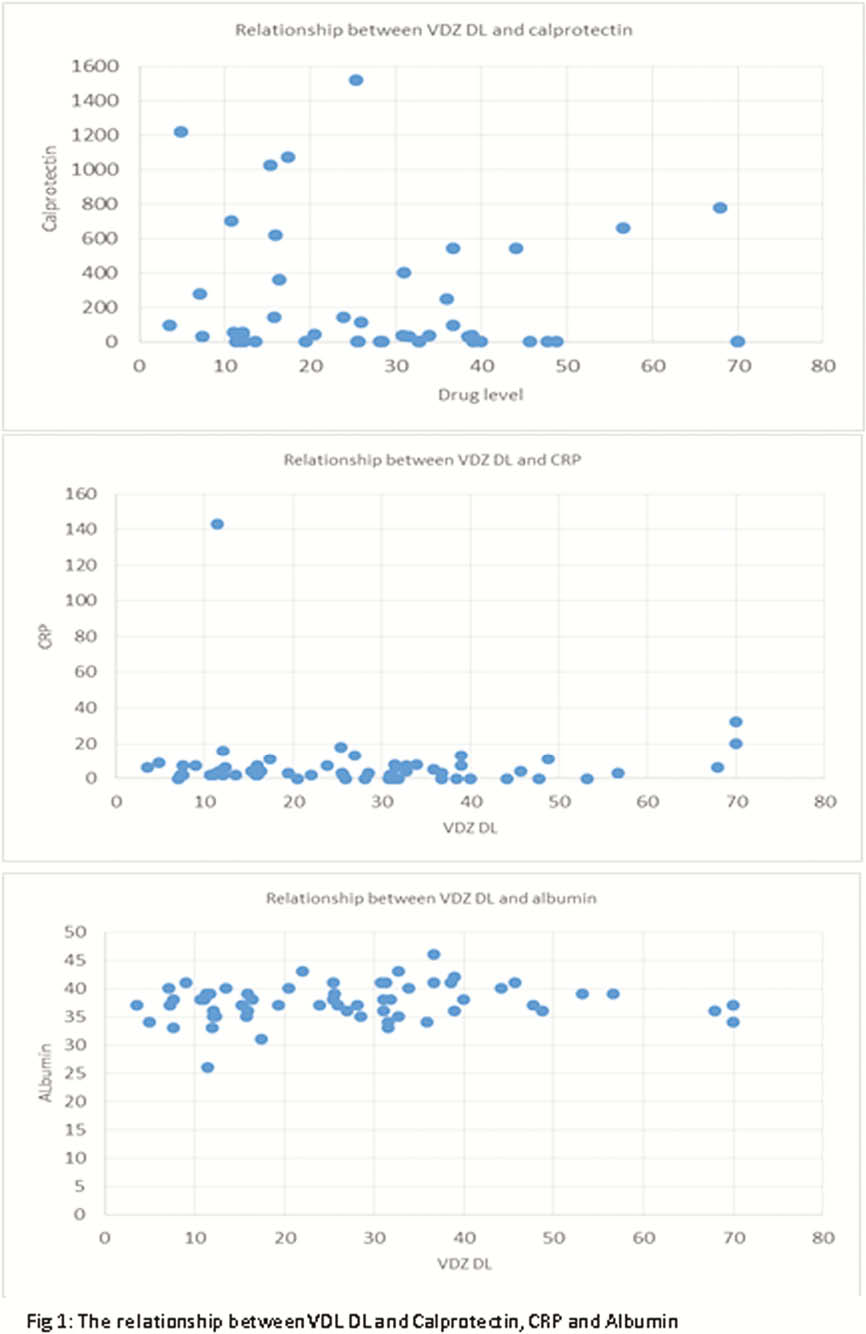
Conclusion
The results from this small cohort do not suggest a relationship between serum VDZ levels and clinical outcomes. Further research in larger cohorts is needed to confirm or refute these findings.
Ben-Horin S, Chowers Y.
Osterman MT, Haynes K, Delzell E,


