P486 Assessing the efficacy of biologics in ucerative colitis: a real-life, observational retrospective multicentre study with propensity score analysis (AURORA): Focus on patients naive to biologics
A. Cassinotti1, N. Mezzina1, D. Di Paolo2, M.V. Lenti3, C. Bezzio4, D. Stradella5, M. Mauri6, V. Zadro1, C. Cortellezzi7, C. Ricci8, V. Casini9, E. Radice10, A. Massari1, S. Ardizzone1, L. Pastorelli2, A. Di Sabatino3, G. Manes4, F. Caprioli11, M. Vecchi11, R. Tari5, P. Occhipinti5, M. Fichera6, P. Invernizzi6, M. Parravicini7, S. Segato7, F. Pace9, P.A. Testoni10, C. Tinelli12, A. De Silvestri12
1Department of Gastroenterology, Luigi Sacco University Hospital, Milan, Italy, 2Gastroenterology and Digestive Endoscopy Unit, IRCCS Policlinico San Donato, San Donato Milanese - Milano, Italy, 3First Department of Medicine, Istituto di Ricovero e Cura a Carattere Scientifico San Matteo Hospital Foundation - University of Pavia, Istituto di Ricovero e Cura a Carattere Scientifico San Matteo Hospital Foundation - University of Pavia, Pavia, Italy, 4Department of Gastroenterology, ASST Rhodense - Rho and Garbagnate Milanese Hospital, Milano, Italy, 5Department of Gastroenterology, ‘Maggiore Della Carità’ Hospital - Novara, Novara, Italy, 6Gastroenterology Division, San Gerardo Hospital- ASST Monza, Monza, Italy, 7ASST Sette Laghi- Ospedale di Circolo Fondazione Macchi, Division of Gastroenterology, Varese, Italy, 8Spedali Civili and University of Brescia, Gastroenterology Section - 1st Medical Clinic, Brescia, Italy, 9Gastroenterology, ASST Bergamo EST-Ospedale Bolognini, Bergamo, Italy, 10Gastroenterology Unit, IRCCS San Raffaele Scientific Institute, Milano, Italy, 11Department of Gastroenterology and Endoscopy, IRCCS Ca’ Granda Foundation - University of Milan, Milano, Italy, 12Fondazione IRCCS Policlinico San Matteo, Servizio di Epidemiologia Clinica e Biometria Direzione Scientifica, Pavia, Italy
Background
Recently, comparative trials among biologics in ulcerative colitis (UC) provided conflicting results on their reciprocal superiority or equivalence. Therefore, in patients naive to biologics, the first-choice biological drug is uncertain.
Methods
In a retrospective, real-life, multicentre inception cohort study involving 11 Italian IBD tertiary centres, all consecutive patients, naive to biologics, treated with adalimumab (ADA), infliximab biosimilar (CTP-13), golimumab (GOL) or vedolizumab (VDZ) after their postmarketing approval (2014–2018) for moderate–severe active UC, were followed up for 1 year or until relapse. All drugs were compared with each other and to naive patients treated with IFX-originator (IFX-O, Remicade) in 2013–2014 as a reference group. A propensity score analysis was performed. The primary endpoint was the 1 year relapse-free, optimisation-free, steroid-free remission, defined as Mayo score ≤2, with bleeding subscore = 0, no relapse after first clinical remission and no optimisation with dose intensification or steroids courses. Multiple further secondary endpoints were analysed (Table 1).
Results
Two hundred ninety-six naive patients (ADA = 56, CTP-13 = 73, GOL = 60, VDZ = 34, IFX-O = 73) were included. The primary end-point was achieved in similar percentages in all groups, irrespective of optimisation. IFX-O and ADA had similar rates of clinical remission achieved once during the follow-up but higher rates than GOL and VDZ. The 1-year relapse rate, however, was lower with VDZ than ADA, GOL and IFX-O. Treatment failure for primary/secondary no response was higher with GOL than IFX-O and ADA. Treatment failures for intolerance were similar among all drugs. CTP-13 performed differently than the originator for some secondary end-points.
Conclusion
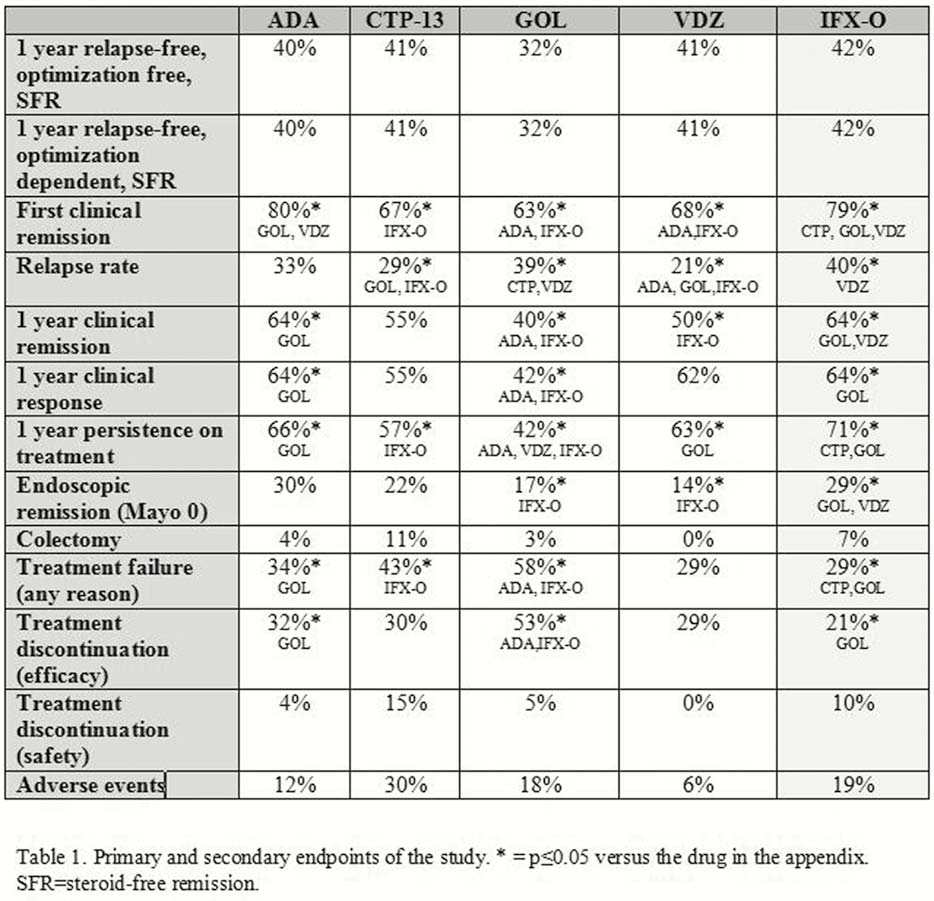
Based on a strict definition of clinical remission, all biologics appear equally effective at 1 year in patients naive to these drugs. IFX originator and ADA appear more effective in the induction phase, while patients responders to VDZ had more prolonged clinical remission. Some differences on secondary questionable outcomes between IFX biosimilar and originator have been observed.


