P496 Efficacy and safety of ustekinumab for chronic antibiotic refractory pouchitis: A Belgian open-label multicentre pilot study
Outtier, A.(1);Louis, E.(2);Dewit, O.(3);Schops, G.(1);Verstockt, B.(1);Sabino, J.(1);Vermeire, S.(1);Ferrante, M.(1);
(1)UZ Leuven, Gastroenterology, Leuven, Belgium;(2)CHU Liège- Sart Tilman, Gastroenterology, Liège, Belgium;(3)Cliniques Universitaires Saint-Luc, Gastroenterology, Bruxelles, Belgium;
Background
Up to 10% of ulcerative colitis patients who undergo a proctocolectomy with ileal pouch-anal anastomosis, will develop chronic antibiotic refractory pouchitis (CARP). As there is a large unmet need in the management of these patients, we evaluated the efficacy and safety of induction therapy with ustekinumab (UST) for this indication.
Methods
We performed a prospective, Belgian, multicentre, open-label study of patients with CARP. Patients received a weight-range-based infusion of UST at baseline (6mg/kg) and one subcutaneous injection of 90mg UST at week 8. The first 4 weeks, ciprofloxacin 500mg BID was added as bridging therapy, but need of antibiotics thereafter was regarded as treatment failure. Patients underwent pouchoscopy at baseline and week 16, with assessment of the modified pouchitis disease activity index (mPDAI). The primary endpoint was the proportion of patients achieving clinically relevant steroid-free remission (mPDAI <5 and reduction by ≥2 points from baseline) at week 16. Secondary endpoints at week 16 were the proportion of patients achieving response (reduction of mPDAI by ≥2 points from baseline), change in symptomatic and endoscopic mPDAI subscore, and change in C-reactive protein (CRP) and faecal calprotectin levels compared to baseline. Descriptive statistics and paired nonparametric tests (Wilcoxon signed-rank) of changes from baseline were performed.
Results
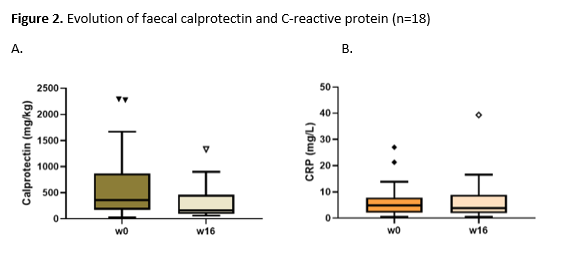
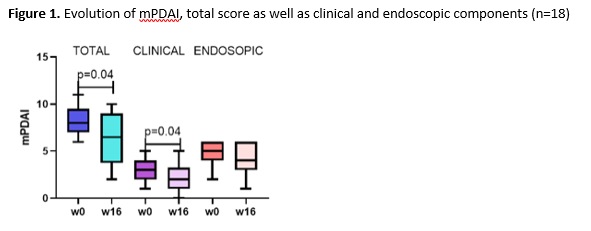
We report the first 18 patients (56% male, median age 41.5 years, median time after surgery 11.2 years) who reached the primary endpoint assessment. Eleven (61.1%) patients had previously been treated with biologics (anti-TNF, n=8; vedolizumab, n=6; tofacitinib, n=1) for CARP (table 1). At week 16, clinically relevant steroid-free remission was reached in 27.8% and response in 50.0% of patients. A significant decrease in total mPDAI (8.0 (7.0-8.3) vs. 6.5 (3.8-9.0), p=0.04) and clinical subscore (3.0 (2.0-4.0) vs. 2.0 (1.0-3.3), p=0.04) but not endoscopic subscore (5.0 (4.0-6.0) vs. 4.0 (3.0-6.0), p=0.1) was observed at week 16 (figure 1). Faecal calprotectin (288.0 (154.8-634.8) vs. 145 (88.3-749.3) mg/kg, p=0.47) and CRP (4.8 (1.9-7.2) vs. 3.9 (1.9-8.9) mg/L, p=0.63) levels did however not decrease significantly (figure 2). Three serious adverse events (hospitalization for subobstruction, worsening pouchitis and choledocholithiasis) were recorded, but were not considered related to UST.
Conclusion
In this open-label pilot study in patients with CARP, induction therapy with UST showed a clinical effect in half of the patients, but was not associated with endoscopic improvement or changes in inflammatory biomarkers. Placebo-controlled studies are needed for further positioning UST in the treatment algorithm of CARP.