P498 Ustekinumab Real World Evidence Study (UndieS): study design with information about positioning and the choice of ustekinumab
Young, D.(1,2);Booker, C.(1);Samaan, M.(3);Limdi, J.(4,5);Kok, K.(6);Donovan, F.(7);Hart, A.(8);Smith, M.(9);Bodger, K.(10);Batchelor, J.(2);Cummings, F.(1,2)*;
(1)University Hospital Southampton NHS Foundation Trust, Department of Gastroenterology, Southampton, United Kingdom;(2)University of Southampton, Faculty of Medicine, Southampton, United Kingdom;(3)Guy’s and St Thomas’ NHS Foundation Trust, Department of Gastroenterology, London, United Kingdom;(4)Northern Care Alliance NHS Foundation Trust NE Sector, Department of Gastroenterology, Manchester, United Kingdom;(5)University of Manchester, School of Medical Sciences, Manchester, United Kingdom;(6)Barts Health NHS Trust, Department of Gastroenterology, London, United Kingdom;(7)St George's University Hospitals NHS Foundation Trust, Department of Gastroenterology, London, United Kingdom;(8)St Marks Hospital and Academic Institute, Department of Gastroenterology, London, United Kingdom;(9)University Hospitals Sussex NHS Foundation Trust, Department of Gastroenterology, Brighton, United Kingdom;(10)University of Liverpool, Department of Health Data Science, Liverpool, United Kingdom; On behalf of the UndieS studygroup.
Background
Strict eligibility criteria of phase 3 trials limit the external validity of their results. Well-designed real-world studies are intended to complement these by describing the effectiveness and safety of these medicines in routine clinical practice. As therapeutic options for the treatment of Crohn’s disease (CD) expands, the ideal sequence of medications, and the influences on this, needs defining.
Methods
UndieS is a phase IV, multi-centre, prospective, observational study of patients with CD initiated on ustekinumab as part of their routine care. 600 participants will be recruited from 22 centres across the UK with a follow-up period of 2 years. Assessments at baseline and weeks 8, 16/20, 56, 80 and 104 include modified Harvey-Bradshaw Index (mHBI), IBD-Control, Treatment Satisfaction Questionnaire for Medication and PRO-2. The primary outcome is steroid-free remission (mHBI ≤ 4) at one year. Secondary outcome measures include describing changes in disease activity, PROs and tolerability.
Results
Recruitment started in March 2020 with 437 patients recruited so far. The summary baseline characteristics are 50% male, median (range) age at consent 41 (18-91) years (14% > 65 years), median mHBI 5 (0-39). The median duration of Crohn’s disease before starting ustekinumab was 8 (0-60) years. Disease location (as per Montreal classification) is: L1: 35%, L2: 20%, L3: 44% and isolated L4: 1%. 24% of participants reported perianal disease. The proportion of participants using concomitant immunomodulators or oral corticosteroids on starting ustekinumab (15% and 11% respectively) is lower than in the UNITI clinical trial program.
59% of participants had a disease activity score suggesting that they would not meet the threshold required for entry into UNITI and suggested by NICE for moderately-to-severely active CD, although 81% of these had evidence of inflammation. Inadequate response to or adverse effects from a previous treatment was a common indication (52% of participants).
32% of the participants are bio-naïve; 64% and 10% have previous exposure to anti-TNF and vedolizumab respectively and 6% have been exposed to both. Details of prior biologic exposure is shown in figure 1. The most common reasons for the specific choice of ustekinumab were intolerance to immunomodulators and concern about infection risk. Bio-naïve patients were older (median 45 vs. 39 years; p=0.02) and had a shorter disease duration (7 vs. 9 years; p=0.04) than those with prior biologic exposure.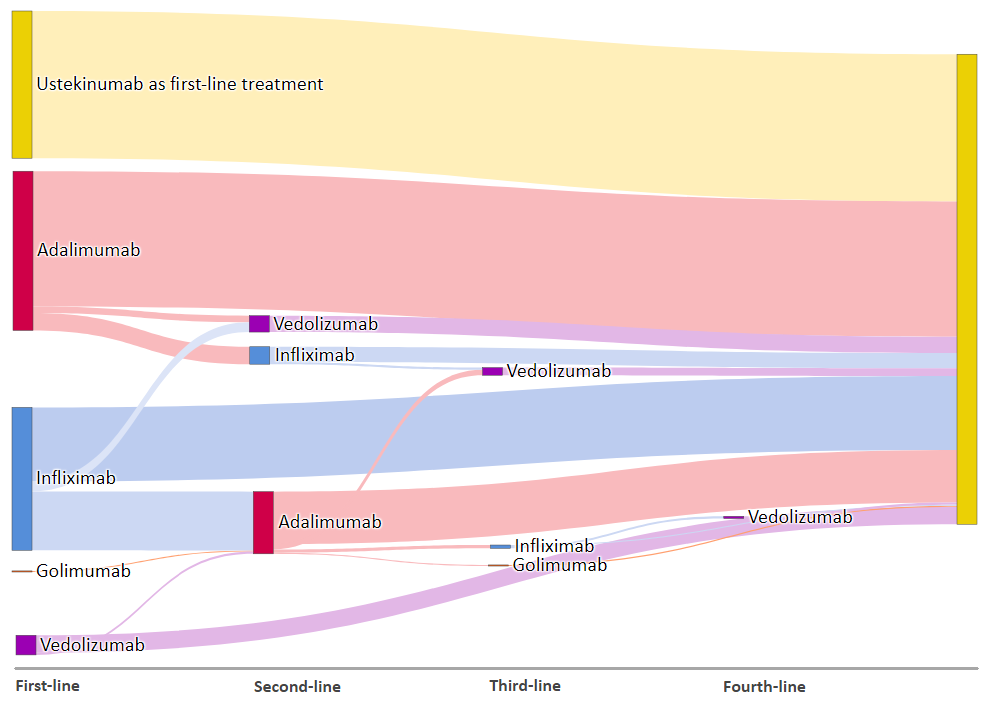
Conclusion
Our cohort is representative of those patients with CD being treated with ustekinumab in a real-world setting. Some important differences between these patients and those recruited to phase 3 clinical trials are identified.


