P499 Objectively assessed disease activity during ustekinumab treatment in a nationwide real-life Crohn’s disease cohort
C.G. af Björkesten1, T. Ilus2, T. Hallinen3, E. Soini3, A. Eberl1, K. Hakala4, M. Heikura5, E. Hirsi6, A. Jussila2, M. Kellokumpu7, R. Koskela8, I. Koskinen9, V. Moilanen10, C. Nielsen11, U. Nieminen1, H. Nuutinen12, M. Heikkinen13, U.M. Suhonen14, J. Tillonen15, K. Utriainen16, I. Vihriälä17, C. Wennerström18,19, A. Borsi20, R. Nissinen21, M. Koivunen21, T. Sipponen1
1University of Helsinki and Helsinki University Hospital, University of Helsinki and Helsinki University Hospital, Helsinki, Finland, 2Tampere University Hospital, Tampere University Hospital, Tampere, Finland, 3ESiOR Oy, ESiOR Oy, Kuopio, Finland, 4Kanta-Häme Central Hospital, Kanta-Häme Central Hospital, Hämeenlinna, Finland, 5North Karelia Central Hospital, North Karelia Central Hospital, Joensuu, Finland, 6South Karelia Central Hospital, South Karelia Central Hospital, Lappeenranta, Finland, 7Lapland Central Hospital, Lapland Central Hospital, Rovaniemi, Finland, 8Oulu University Hospital, Oulu University Hospital, Oulu, Finland, 9Central Finland Central Hospital, Central Finland Central Hospital, Jyväskylä, Finland, 10Satakunta Central Hospital, Satakunta Central Hospital, Pori, Finland, 11Vaasa Central Hospital, Vaasa Central Hospital, Vaasa, Finland, 12Turku University Hospital, Turku University Hospital, Turku, Finland, 13Kuopio University Hospital, Kuopio University Hospital, Kuopio, Finland, 14Kainuu Central Hospital, Kainuu Central Hospital, Kajaani, Finland, 15Päijät-Häme Central Hospital, Päijät-Häme Central Hospital, Lahti, Finland, 16Turku University Hospital- Salo Hospital, Turku University Hospital- Salo Hospital, Salo, Finland, 17Central Ostrobothnia Central Hospital, Central Ostrobothnia Central Hospital, Kokkola, Finland, 18Janssen Cilag AB, Medical Affairs, Solna, Sweden, 19Statens Serum Institut, Department of Epidemiology Research, Copenhagen, Denmark, 20Janssen Cilag Limited, emea hemar, High Wycombe, UK, 21Janssen Cilag Oy, Medical Affairs, Espoo, Finland
Background
Real-life long-term evidence on ustekinumab treatment response in patients with Crohn’s disease (CD) is scarce. We performed a retrospective non-interventional nationwide chart review study of dosing and long-term clinical outcomes in Finnish CD patients treated with ustekinumab (FINUSTE2, EUPAS30920).
Methods
FINUSTE2 involved 17 Finnish centres. Eligible patients were adults with CD, receiving an intravenous first dose of ustekinumab during 2017 or 2018. Disease activity data, such as C-reactive protein (CRP), faecal calprotectin (fCal), and the Simple Endoscopic Score for Crohn’s disease (SES-CD) were collected at baseline, 16 weeks, and one year from treatment initiation. A local gastroenterologist at each centre collected the data from health records in an electronic standardised health questionnaire.
Results
One hundred and fofty-five patients (48% female) with a mean age of 44 and disease duration of 14 years initiated ustekinumab treatment for CD. Table 1 summarises patient characteristics at baseline. After induction consisting of one intravenous dose and one to two subcutaneous doses at 8 to 16 weeks, 140 patients (93%) continued to maintenance treatment with subcutaneous ustekinumab. Of 93 patients with a follow-up of at least one year, 77 were still on ustekinumab. During ustekinumab treatment, SES-CD (10 at baseline, 3 at 1 year, medians,
| Age, years | 155 | 44.5 (15.3) | |
| Disease duration, years | 1481 | 13.9 (10.6) | |
| SES-CD | 431 | 11.4 (5.4) | |
| Gender, female | 74 | 47.7 | |
| Smoking2 | 32 | 22.5 | |
| Prior biologic treatment for CD | 150 | 96.8 | |
| Lack of efficacy of prior biologic | 130 | 83.8 | |
| Side effects of prior biologic | 50 | 32.2 | |
| Prior CD-related surgery | 92 | 59.4 | |
| Montreal classification | |||
| A1 | 36 | 23.2 | |
| A2 | 87 | 56.1 | |
| A3 | 32 | 20.7 | |
| L1 or L1+L4 or L4 | 39 | 25.2 | |
| L2 | 26 | 16.8 | |
| L3 or L3+L4 | 90 | 58.1 | |
| B1 | 48 | 31.0 | |
| B2 | 84 | 54.2 | |
| B3 | 23 | 14.8 | |
| Perianal | 49 | 31.6 |
1Number depicts patients with available data, 2Smoking status unknown for 13 patients. SES-CD: Simple Endoscopic Score for Crohn’s disease; CD: Crohn’s disease.
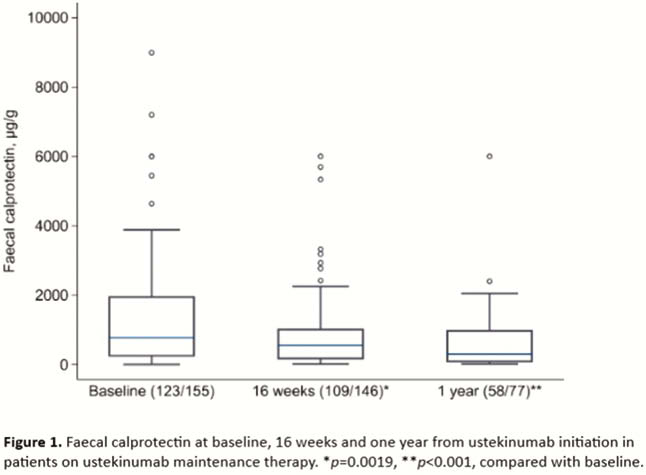
Conclusion
In this nationwide real-life long-term follow-up study, covering all major centres in Finland, ustekinumab treatment of patients with highly refractory and long-standing CD effectively reduced inflammatory activity, assessed by endoscopy, CRP, and fCal.


