P507 Ustekinumab: Medium-term outcomes from a UK multicentre real-world cohort
R. Gadhok1, K. Fragkos2, S. Honap3, J. Hassan2, L. Whiteley2, A. Ibarra1, N. Burgess1, R. Vega2, E. Seward2, S. Mehta2, S. McCartney2, S. Bloom2, G. Parkes1, P. Irving3, J.O. Lindsay1, K.B. Kok1, F. Rahman2
1Gastroenterology, Royal London Hospital- Barts Health NHS Trust, London, UK, 2Gastroenterology, University College London Hospital NHS Trust, London, UK, 3Gastroenterology, Guys and St Thomas’ Hospital NHS Trust, London, UK
Background
Ustekinumab is effective at inducing and maintaining remission of Crohn’s disease (CD) in clinical trials. However real-world practice may vary regarding use of concomitant immunomodulator (IM), dosing schedule and patient selection. We present the largest UK real-world, multi-centre study of effectiveness.
Methods
The cohort comprised adult patients initiated on ustekinumab for CD from October 2016–18 at 3 tertiary London centres. Clinical endpoints were (i) remission (Harvey Bradshaw Index (HBI) ≤4) (ii) response, (reduction in HBI of ≥3 or sustained HBI≤4 points) at 3, 6 and 12 months. Biological endpoints were remission and response (CRP <5mg/l in patients with a baseline CRP>5mg/l, and 50% reduction in CRP, respectively).
Results
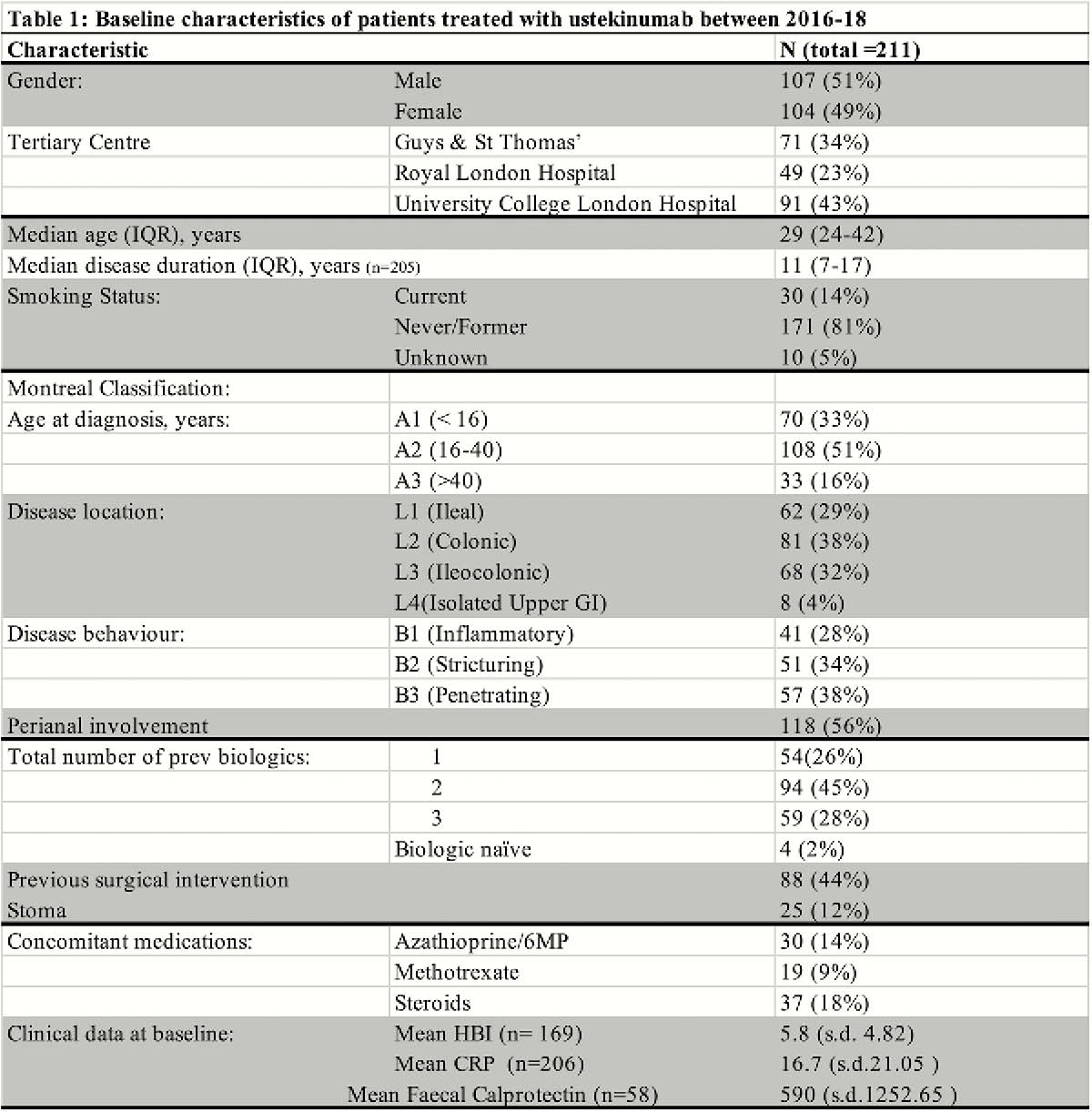
211 patients were included (Table 1), of whom 207 (98%) were biologic exposed and 59 (28%) had failed 3 biologics. All patients received i.v. induction and 203 (96%) received a s.c. dose at week 8. At 6 and 12 months, 133 (74.8%) and 109 (73.6%) patients, respectively, were on 8 weekly dosing. Dosing schedule did not impact clinical and biological outcome at 6 and 12 months.
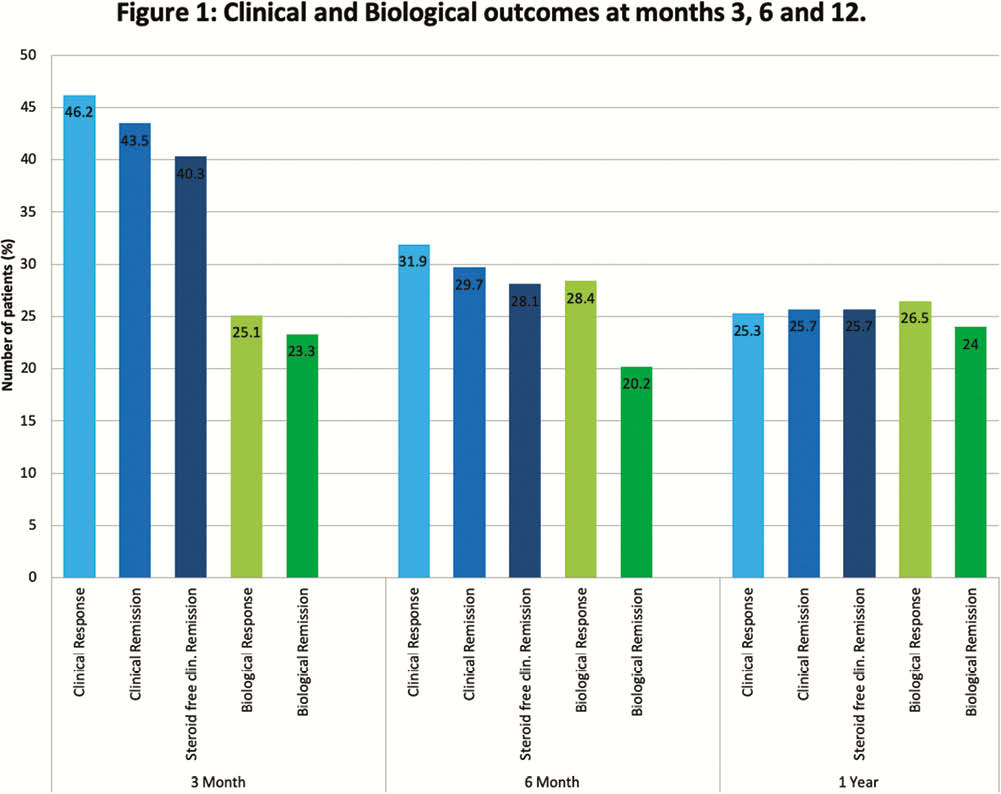
Discontinuation occurred in 4 (1.9%), 20 (9.5%) and 50 (23.7%) patients by 3, 6 and 12 months respectively. Reasons for stopping included: drug reactions (4(1.9%)), partial response (4 (1.9%)), loss of response (10 (4.7%)) and primary non-response. (24 (11.4%)). Clinical and biological outcomes at 3, 6 and 12 months are shown in Figure 1. Adverse events occurred in 27 (12.8%) patients. IM were prescribed in 49 (23%) at baseline and continued in 46 (21.8%), 36 (17.1%), 27 (12.8%) at 3, 6 and12 months respectively. There was no significant difference in median persistence (months) on ustekinumab between patients who were on an IM at baseline vs. those not (15.2 (95% CI 0–32.2) vs. 23.3 (17.3–29.4) months,
Conclusion
Ustekinumab is effective in a real-world cohort. In keeping with trial data, we did not find evidence of improved outcomes with the use of a concomitant IM, nor differential outcomes when considering perianal and isolated colonic disease.


