P509 Influence of concomitant immunosuppresives in retention rate in Crohn´s Disease patients under ustekinumab in the SUSTAIN Study
Chaparro, M.(1);Bastón Rey, I.(2);Fernández-Salgado, E.(3);González García, J.(4);Ramos, L.(5);Diz-Lois Palomares, M.T.(6);Argüelles, F.(7);Iglesias Flores, E.(8);Cabello, M.(9);Rubio Iturria, S.(10);Núñez Ortiz, A.(11);Charro, M.(12);Ginard, D.(13);Dueñas Sadornil, C.(14);Merino Ochoa, O.(15);Busquets, D.(16);Iyo, E.(17);Gutiérrez Casbas, A.(18);Ramírez de la Piscina, P.(19);Boscá-Watts, M.M.(20);Arroyo, M.(21);García, M.J.(22);Hinojosa, E.(23);Gordillo, J.(24);Martínez Montiel, P.(25);Velayos Jiménez, B.(26);Quílez Ivorra, C.(27);Vázquez Morón, J.M.(28);Huguet, J.M.(29);González Lama, Y.(30);Muñagorri Santos, A.I.(31);Amo, V.M.(32);Martín Arranz, M.D.(33);Bermejo, F.(34);Martínez Cadilla, J.(35);Fradejas Salazar, P.(36);Novella, C.(37);Vispo, E.(37);Barreiro-de Acosta, M.(2);Gisbert, J.P.(1);
(1)Hospital Universitario de La Princesa- Instituto de Investigación Sanitaria Princesa IIS-IP- Universidad Autónoma de Madrid and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas CIBERehd, Gastroenterology Department, Madrid, Spain;(2)Complejo Hospitalario Universitario de Santiago, Gastroenterology Department, Santiago de Compostela, Spain;(3)Complejo Hospitalario de Pontevedra, Gastroenterology Department, Pontevedra, Spain;(4)Hospital Público Comarcal la Inmaculada, Gastroenterology Department, Almería, Spain;(5)Hospital Universitario de Canarias, Gastroenterology Department, Tenerife, Spain;(6)Hospital Universitario A Coruña, Gastroenterology Department, A Coruña, Spain;(7)Hospital Universitario Virgen Macarena and Universidad de Sevilla, Gastroenterology, Sevilla, Spain;(8)Hospital Universitario Reina Sofía, Gastroenterology Department, Córdoba, Spain;(9)Hospital Universitario Virgen de Valme, Gastroenterology Department, Sevilla, Spain;(10)Complejo Hospitalario de Navarra, Gastroenterology Department, Pamplona, Spain;(11)Hospital Universitario Virgen del Rocío, Gastroenterology Department, Sevilla, Spain;(12)Hospital de Barbastro, Gastroenterology Department, Barbastro, Spain;(13)Hospital Universitario Son Espases, Gastroenterology Department, Palma de Mallorca, Spain;(14)Hospital San Pedro de Alcántara, Gastroenterology Department, Cáceres, Spain;(15)Hospital Universitario Cruces, Gastroenterology Department, Barakaldo, Spain;(16)Hospital Universitari de Girona Doctor Josep Trueta, Gastroenterology Department, Girona, Spain;(17)Hospital Comarcal de Inca, Gastroenterology Department, Inca, Spain;(18)Hospital General Universitario de Alicante- Instituto de Investigación Sanitaria y Biomédica de Alicante ISABIAL- Centro de Investigación Biomédica en Red de Enfermedades Hepáticas Digestivas CIBERehd- Madrid, Gastroenterology Department, Alicante, Spain;(19)Hospital Universitario de Araba-Txagorritxu, Gastroenterology Department, Araba-Txagorritxu, Spain;(20)Hospital Clínico de Valencia, Gastroenterology Department, Valencia, Spain;(21)Hospital Clínico Universitario Lozano Blesa, Gastroenterology Department, Zaragoza, Spain;(22)Hospital Universitario Marqués de Valdecilla- IDIVAL, Gastroenterology, Santander, Spain;(23)Hospital de Manises, Gastroenterology Department, Manises, Spain;(24)Hospital de la Santa Creu i Sant Pau, Gastroenterology Department, Barcelona, Spain;(25)Hospital Universitario 12 de Octubre, Gastroenterology Department, Madrid, Spain;(26)Hospital Universitario de Valladolidd, Gastroenterology Department, Valladolid, Spain;(27)Hospital Marina Baixa, Gastroenterology Department, Villajoyosa, Spain;(28)Hospital Universitario Juan Ramón Jiménez, Gastroenterology Department, Huelva, Spain;(29)Hospital General Universitario de Valencia, Gastroenterology Deparment, Valencia, Spain;(30)Hospital Universitario Puerta de Hierro, Gastroenterology Department, Majadahonda, Spain;(31)Hospital Universitario de Donostia, Gastroenterology Department, San Sebastián, Spain;(32)Hospital Regional de Málaga, Gastroenterology Department, Málaga, Spain;(33)Hospital Universitario de La Paz- Instituto de Investigación Sanitaria del Hospital La Paz IdiPaz- Facultad de Medicina- Universidad Autónoma de Madrid, Gastroenterology Department, Madrid, Spain;(34)Hospital Universitario de Fuenlabrada- Instituto de Investigación Sanitaria del Hospital La Paz IdiPaz, Gastroenterology Department, Madrid, Spain;(35)Hospital Álvaro Cunqueiro, Gastroenterology Department, Vigo, Spain;(36)Hospital Virgen de la Concha, Gastroenterology Department, Zamora, Spain;(37)Janssen Medical Department, Gastroenterology Department, Madrid, Spain
Background
Whether to use biologic treatment for inflammatory bowel disease as monotherapy or in combination with immunosuppressives has been a matter of debate in the last years. We aimed to evaluate if immunosuppressants influenced the retention rate of ustekinumab in Sustain, the largest study evaluating its use in clinical practice, having the longest follow-up period reported to date.
Methods
Retrospective, multicentre study (>60 sites) including patients with active Crohn´s Disease (CD) [(Harvey-Bradshaw (HBI)>4)] who received ≥1 dose of ustekinumab intravenously before July 2018. Data on concomitant immunomodulatory therapy (if any) with azathioprine, mercaptopurine, methotrexate or others at the start of ustekinumab treatment and during follow-up were documented. Clinical remission was defined as HBI≤4 and response as ≥3 points decrease from baseline. Loss of response (LoR) was defined as reappearance of symptoms that led to intensifying the treatment dose, adding another medication to control CD, switching or surgery in patients with short-term remission. The retention rate in patients on ustekinumab receiving or not immunosuppressive treatment was evaluated by descriptive analysis and Kaplan-Meier survival curves. Predictive factors were assessed by Cox-regression. Data quality was assured by remote monitoring.
Results
463 CD patients were included. Prior CD treatments to ustekinumab were collected: the majority of patients received steroids, immunosuppressants and biologics. Figure 1 shows the percentage of patients on treatment with each type of drug and whether or not they continued to receive it when they started ustekinumab, in the case of steroids and immunosuppressants.
Reasons for discontinuation of previous immunosuppressive treatments for CD were as follows: failure (37.5%), sustained remission (5.8%), adverse event (47.6%), medical decision (4%), other reasons (5.1%).
During ustekinumab treatment, 163 patients (35.2%) received immunosuppressants (listed in figure 2). Sixty-six (14.3%) patients had their immunosuppressant withdrawn during treatment.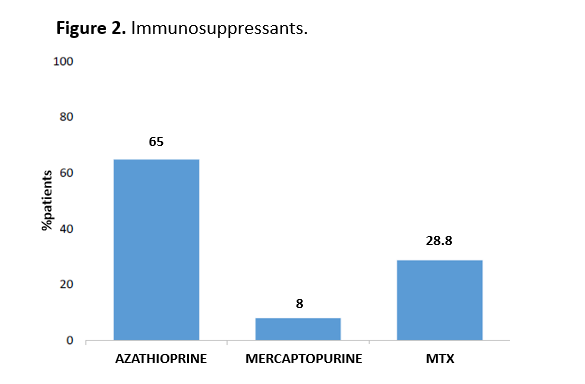
Retention was observed in 77.9% of patients with concomitant immunosuppressive therapy versus 76.3% of patients without. There weren´t statistically significant differences between the two populations (p=0.712). Figure 3.
.png)
The patient-year discontinuation rate for patients who didn´t receive concomitant immunosuppressants was 17.5 versus 18.9 in those who received them.
Conclusion
No effectiveness differences were seen by adding immunosuppressants to ustekinumab, reinforcing the low immunogenic profile of this drug and reducing the risk of additional side effects and toxicity.


