P517 A randomised, placebo-controlled pilot trial of faecal microbiota transplantation for paediatric Crohn’s disease
L. Hill1,2, J. Popov1,3, E. Hartung1, M. Moshkovich1,4, M. Figueiredo1,4, N. Pai1,5, McMaster Paediatric FMT Research Program
1Division of Gastroenterology and Nutrition, Department of Paediatrics- McMaster University, Hamilton, Canada, 2Department of Exercise Science and Sports Medicine, University of Cape Town, Cape Town, South Africa, 3College of Medicine and Health, University College Cork, Cork, Ireland, 4Faculty of Health Sciences, McMaster University, Hamilton, Canada, 5Farncombe Family Digestive Health Research Institute, Department of Medicine- McMaster University, Hamilton, Canada
Background
The role of faecal microbiota transplant (FMT) in Crohn’s disease (CD) remains unclear. Small, open-label case series have shown high rates of clinical remission but protocols have varied across studies, and no randomised controlled trials (RCT) have been performed. We present a protocol for the first pilot RCT of FMT in paediatric CD patients, using a novel colonoscopic + oral capsular intervention that uses fresh-frozen and prepared, lyophilised donor stool. We will measure improvements in clinical disease activity, inflammatory biomarkers, endoscopic markers of mucosal inflammation, as well as, assess key aspects of trial feasibility.
Methods
Patients 3–17 years with active CD, on stable medication doses for 4 weeks are eligible. Patients will have an initial colonoscopy during which they will receive an infusion into the terminal ileum of normal saline (placebo) or prepared healthy donor stool (RBX2660; Rebiotix, USA) (active). This will be followed by 6-weeks of bi-weekly oral capsular therapy, containing methylcellulose (placebo) or lyophilised healthy adult donor stool (RBX7455; Rebiotix, USA). Randomisation is 2:1 to active and placebo arms (
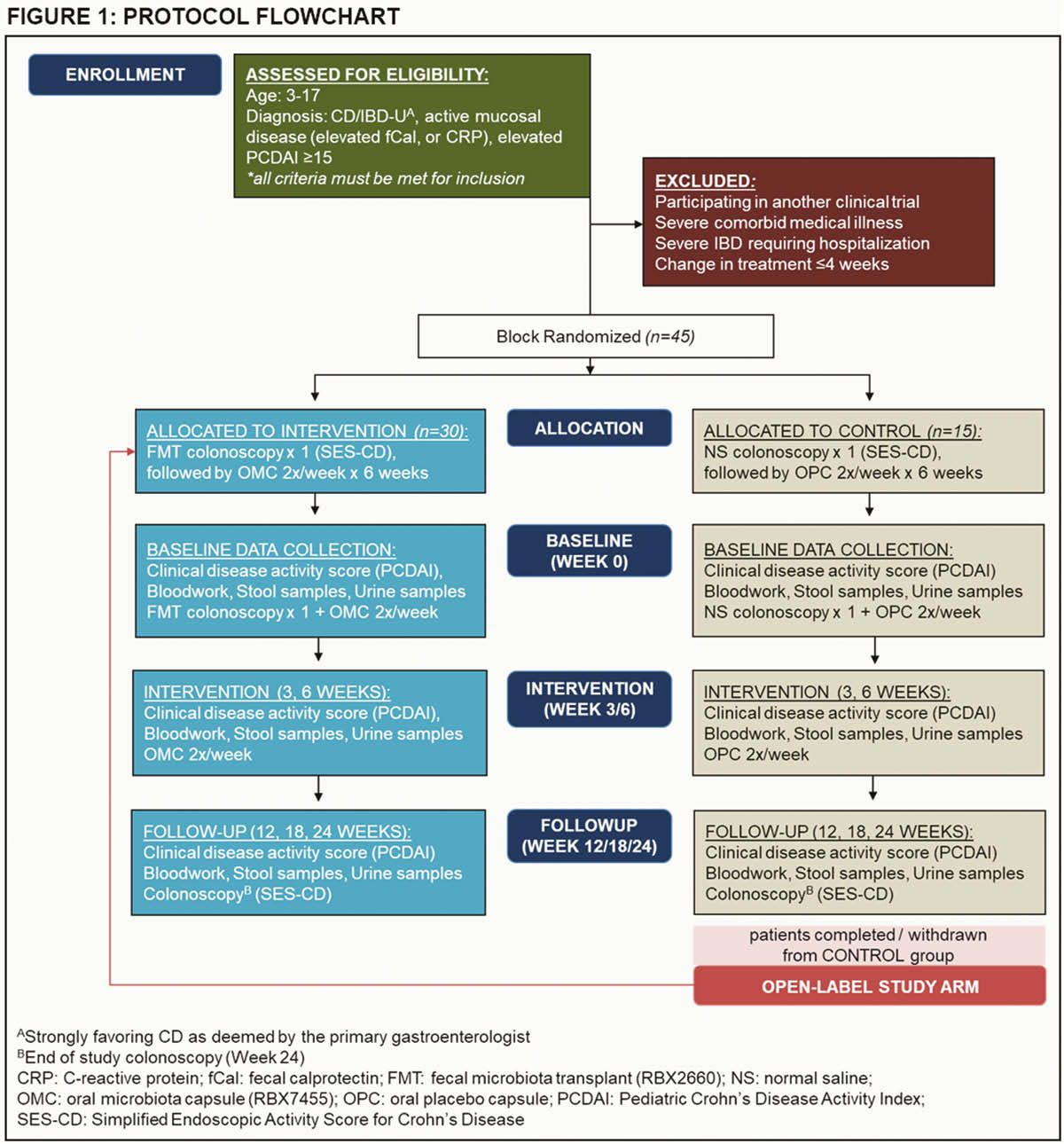
Results
Study feasibility will be assessed by: rate of recruitment, completion of sample collections, and frequency and type of adverse events. Clinical outcomes will be measured serially using: Paediatric Crohn’s Disease Activity Index (PCDAI), faecal calprotectin, blood inflammatory markers, and Simple Endoscopic Score for Crohn’s Disease (SES-CD) at baseline and end-of-study. Stool 16s rRNA, metagenomics, and urine metabolomics profiles will be performed on collected stool and urine samples. Patients who complete the placebo arm will be eligible to enter an open-label extension study.
Results will be measured through ITT and PP analyses. Proportions and percentages will be reported on feasibility outcomes; odds ratios, mean differences and 95% confidence intervals will be reported on clinical outcomes as preliminary estimates of efficacy.
Conclusion
This is the first pilot RCT of FMT in the treatment of CD. Recruitment commenced April 2019 and 13 patients have met screening criteria, with 5 enrolled thus far. This study is novel for its focus on paediatric CD patients, and its use of a combined oral + colonoscopic FMT delivery method. The results of this trial will offer preliminary estimates of efficacy for FMT-based therapies in CD, and may support expansion to a future larger multicenter paediatric RCT using our validated study protocol.


