P525 Real-world effectiveness and safety of ustekinumab in patients with ulcerative colitis: a systematic review and meta-analysis
Taxonera, C.(1)*;Olivares, D.(1);Olga N, L.G.(2);Alba, C.(1);
(1)Hospital Clínico San Carlos, IBD Unit- Department of Gastroenterology, Madrid, Spain;(2)Hospital Clínico San Carlos, Department of Gastroenterology, Madrid, Spain;
Background
Knowledge of the real-world effectiveness and safety of tofacitinib for ulcerative colitis (UC) is relevant to confirm the benefit observed in clinical trials. The aim of this systematic review was to summarize reported evidence on the real-world outcomes of ustekinumab for UC and to conduct a meta-analysis of effectiveness and safety data.
Methods
A systematic search was conducted in electronic databases PubMed, Web of Science, EMBASE, and Science Direct, and conference proceedings until 15 September 2022 for real-world studies evaluating ustekinumab for UC. A random-effects meta-analysis model was used to calculate the pooled rates of clinical and safety outcomes. We evaluated the outcomes at week 8, week 12 to 16, month 6, and month 12. Need for colectomy and adverse events (AE) were reported as percentages and incidence rates (IRs) per 100 patients-year (PY) of exposure.
Results
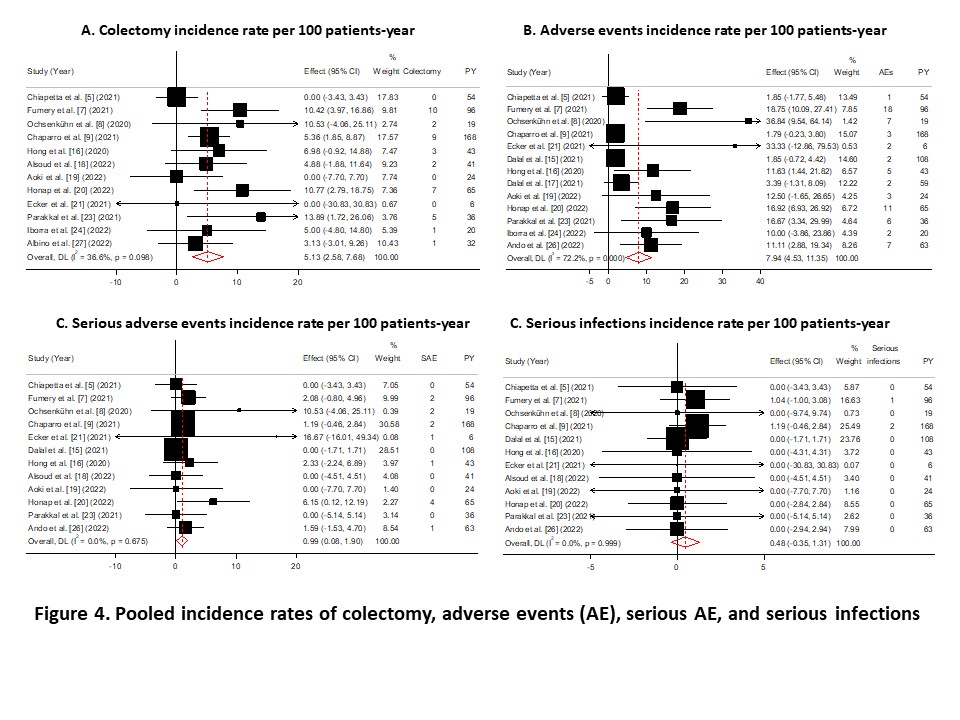
Of the 254 citations identified, 19 studies were included with 3786 patients. Sixteen studies reported prior exposure to biologics or JAK inhibitors and included 1003 patients, of whom 92.3% were previously treated with any biologic, 61.1% with both anti-TNF and vedolizumab, and 16.4% with any biologic and tofacitinib (Figure 1). Remission was achieved in 45.4% of patients at week 8 (95% confidence interval 30.1-60.6%), 43.8% (38.4-49.2%) at weeks 12 to 16, 44.6% (35.9-53.3%) at month 6, and 50.6% (36.3-64.8%) at month 12 (Figure 2). Response was achieved in 61.2%, 59.4%, 65.2%, and 76.8% of patients at week 8, weeks 12 to 16, month 6, and month 12, respectively (Figure 3). Corticosteroid-free remission was achieved in 18.7%, 36.8%, 34.5%, and 39% of patients at week 8, weeks 12 to 16, month 6, and month 12, respectively. Endoscopic improvement was achieved in 29.9%, 24.3% %, and 58.2% of patients at weeks 12 to 16, month 6, and month 12, respectively. Almost 30% of the patients needed dose escalation, which was effective in 40% of these patients. The pooled rate of persistence with ustekinumab was 76.9% and 73.3% at month 9 and month 12, respectively. The IRs of colectomy, AE, serious AE, and serious infections were 5.1, 7.9, 1 and 0.5 per 100 PY, respectively (Figure 4).


Conclusion
The results of this systematic review and meta-analysis of real-world studies confirm the effectiveness of ustekinumab in a highly treatment-refractory population of patients with active moderate-to-severe UC. The acceptable safety data presented here support the positive long-term benefit-risk profile of ustekinumab in the treatment of UC. These real-world effectiveness and safety results were consistent with clinical trials and provide a broader perspective that can be used to aid treatment decisions in a more heterogeneous clinical setting.


