P526 Body mass index and response to TNF-α inhibitors in inflammatory bowel disease
F. Nordin1, B. Shadbolt2, K. Subramaniam1,3
1Gastroenterology and Hepatology Unit, Canberra Hospital, Canberra, Australia, 2Health Analytics Research Centre, ACT Health, Canberra, Australia, 3Australian National University Medical School, Australian National University, Canberra, Australia
Background
Obesity is an emerging issue in the care of patients with inflammatory bowel disease (IBD). The aim of the study was to evaluate whether the response to TNF-α inhibitors (infliximab and adalimumab) could be influenced by body mass index (BMI) in IBD. Unlike adalimumab (ADA), a recombinant humanised IgG antibody against (TNF-α), the dosing of infliximab (IFX); a monoclonal chimeric antibody is weight-based.
Methods
We identified a cohort of patients with IBD at a single centre, naïve to biologic therapy and stratified them according to their weight and BMI. The primary outcome is the first occurrence of loss of response defined as clinical deterioration requiring hospitalisation, surgery, corticosteroid use, dose escalation or discontinuation of therapy and/or evidence of activity on endoscopy. Patients were followed up for 4 years. Multivariate analysis with logistic regression were used to compare variables.
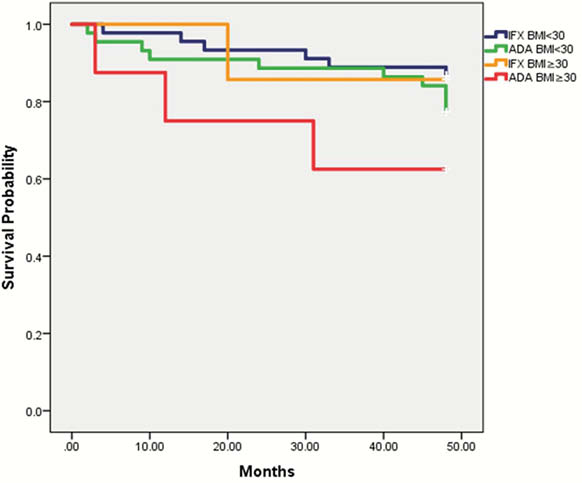
Results
There was a total of 104 IBD patients; 51% (
In terms of loss of response, type of drug (IFX/ADA) was not found to be a significant risk factor (
Conclusion
BMI appears to be important in predicting loss of response in IBD however we found no overall association between increased BMI and accelerated loss of response. Further larger studies are needed to evaluate the relationship of BMI and loss of response to ADA.


