P526 Measurement of thiopurine metabolites concentrations after one week of treatment does not predict treatment failure in Inflammatory Bowel Disease patients
DebenMSc, D.(1);Van Moorsel, F.(2,3);Creemers, R.(4,5);Winkens, B.(6);Bus, P.(7);Pierik, M.(5);Simsek, M.(8);De Boer, N.(8);Wong, D.(1);Van Bodegraven, A.(4,8);
(1)Zuyderland MC, Dept. of Clinical Pharmacy- Clinical pharmacology and Toxicology, Sittard-Geleen, The Netherlands;(2)Jeroen Bosch Hospital, Dept. of Pharmacy, 's-Hertogenbosch, The Netherlands;(3)Bernhoven Hospital, Dept. of Clinical Pharmacy, Uden, The Netherlands;(4)Zuyderland MC, Dept. of Gastroenterology- Geriatrics- Internal and Intensive Care Medicine Co-MIK, Sittard-Geleen, The Netherlands;(5)Maastricht University Medical Center, Dev. of Gastroenterology and Hepatology, Maastricht, The Netherlands;(6)Maastricht University Medical Center, Dept. of methodology and statistics, Maastricht, The Netherlands;(7)Laurentius Hospital, Dept. of Gastroenterology and Hepatology, Roermond, The Netherlands;(8)Amsterdam University Medical Center, Dept. of Gastroenterology and Hepatology, Amsterdam, The Netherlands;
Background
Conventional thiopurines such as azathioprine (AZA) and mercaptopurine (MP) remain drugs of choice in maintaining remission and corticosteroid sparing in inflammatory bowel disease (IBD). Early discontinuation of thiopurine therapy occurs frequently, due to slow onset of effect and a relatively high frequency of early adverse drug reactions (ADR). Some ADR such as hepatotoxicity and gastro-intestinal complaints are associated with the thiopurine metabolite concentrations 6-thioguanine nucleotides (6-TGN) or 6-methylmercaptopurine ribonucleotides (6-MMPR), in particular elevated 6-MMPR concentrations or unfavorable 6-MMPR/6-TGN ratios.
Early measurement of thiopurine metabolites may help to optimize thiopurine therapy in IBD. The aim of this study was to assess the predictive value of thiopurine metabolite measurement one week after thiopurine therapy initiation on discontinuation during the first three months of therapy.
Methods
This was a multicenter prospective observational study in the Netherlands. Consecutive IBD patients who initiated thiopurine therapy were included and measurement of thiopurine metabolites was performed after 7 days (6 – 8) of treatment (T1). Patients were monitored for 12 weeks to document occurrence of ADR and early treatment discontinuation. Switching to an alternative thiopurine or adding allopurinol (as co-medication) was also considered as treatment discontinuation (to prevent ADR). 6-MMPR concentrations and 6-MMPR/6-TGN ratios were separately analyzed (Mann-Whitney U-tests) regarding therapy discontinuation within 12 weeks. P-values of ≤0.05 were considered statistically significant.
Results
In total, 255 patients were included, of which 83 were excluded due to either missing blood samples on T1 (n=54), dose adjustments within 12 weeks (n=25) or a delayed T1 measurement (n=4). Of the remaining 172 patients, 79 patients were male (46%), 102 patients had Crohn’s disease (59%) and 157 patients were treated with MP (91%). In total, 88 (51%) patients continued thiopurine therapy and 84 patients (49%) discontinued therapy within 12 weeks. Median 6-MMPR concentrations (1,295 versus 1,589 pmol x 108 red blood cells) and 6-TGN/6-MMPR ratios (8.8 versus 11.4) did not differ significantly between patients who continued versus discontinued therapy within 12 weeks (Figures 1 and 2).
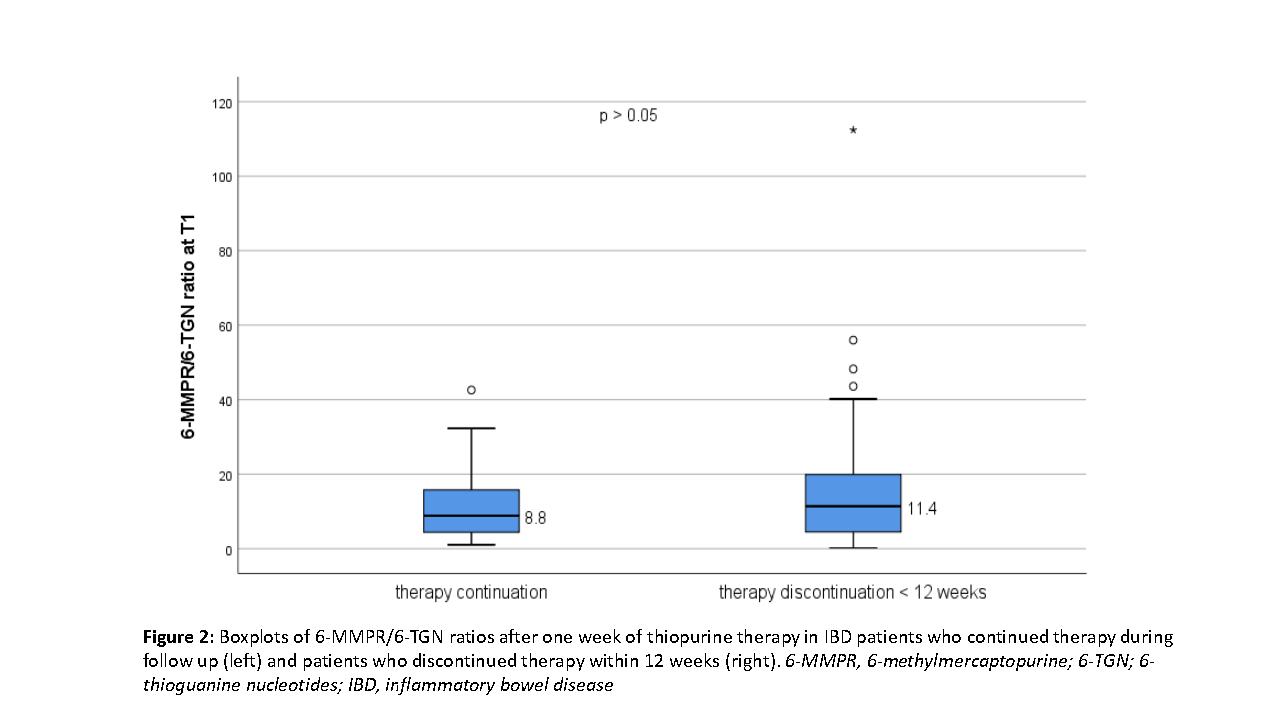
Conclusion
In this study thiopurine metabolites at T1 did not relate to discontinuation of thiopurine therapy within 12 weeks. We will conduct additional pharmacodynamic studies to corroborate our hypothesis that early metabolite measurements may be of individual patients' benefit.


