P527 Investigating fatigue in vedolizumab-treated patients with ulcerative colitis or Crohn’s disease from a Belgian registry
Louis , E.(1);Muls , V.(20);Bossuyt , P.(2);Colard , A.(3);Nakad , A.(4);Baert , D.(5);Mana , F.(6);Caenepeel , P.(7);Vanden Branden , S.(8);Vermeire , S.(9);D'Heygere , F.(10);Strubbe , B.(11);Cremer , A.(12);Setakhr , V.(13);Baert , F.(14);Vijverman , A.(15);Coenegrachts , J.L.(16);Flamme , F.(17);Hantson , A.(18);Bennett , D.(19);Van Gassen , G.(18);Hantsbarger , G.(19);
(1)University Hospital CHU of Liège, Department of Gastroenterology, Liège, Belgium;(2)Imeldaziekenhuis, Department of Gastroenterology, Bonheiden, Belgium;(3)Hospital CHC, Department of Gastroenterology, Liège, Belgium;(4)CHwapi Notre Dame, Department of Gastroenterology, Tournai, Belgium;(5)Maria Middelares Hospital, Department of Gastroenterology, Ghent, Belgium;(6)Clinique St. Jean, Department of Gastroenterology, Brussels, Belgium;(7)Ziekenhuis Oost Limburg, Department of Gastroenterology, Genk, Belgium;(8)Onze Lieve Vrouwziekenhuis, Department of Gastroenterology, Aalst, Belgium;(9)University Hospitals Leuven, Department of Gastroenterology, Leuven, Belgium;(10)AZ Groeninge Hospital, Department of Gastroenterology, Kortrijk, Belgium;(11)AZ St Lucas, Department of Gastroenterology, Gent, Belgium;(12)Hopital Universitaire Erasme, Department of Gastroenterology, Brussels, Belgium;(13)CHU UCL Namur site Sainte Elisabeth, Department of Gastroenterology, Brussels, Belgium;(14)AZ Delta, Department of Gastroenterology, Roeselare, Belgium;(15)Hospital CHR de la Citadelle, Department of Gastroenterology, Liège, Belgium;(16)Jessa Ziekenhuis- Hasselt, Department of Gastroenterology, Hasselt, Belgium;(17)CHU Ambroise Paré, Department of Gastroenterology, Mons, Belgium;(18)Takeda, Department of Gastroenterology, Brussels, Belgium;(19)Takeda, Department of Gastroenterology, Cambridge, United States;(20)SaintPierre University Hospital, Department of Gastroenterology, Brussels, Belgium
Background
Vedolizumab (VDZ) has demonstrated remission in ulcerative colitis (UC) and Crohn’s disease (CD), but its impact on patient (pt) fatigue is not well understood. Herein we report interim fatigue analysis data from a Belgian registry of VDZ-treated pts.
Methods
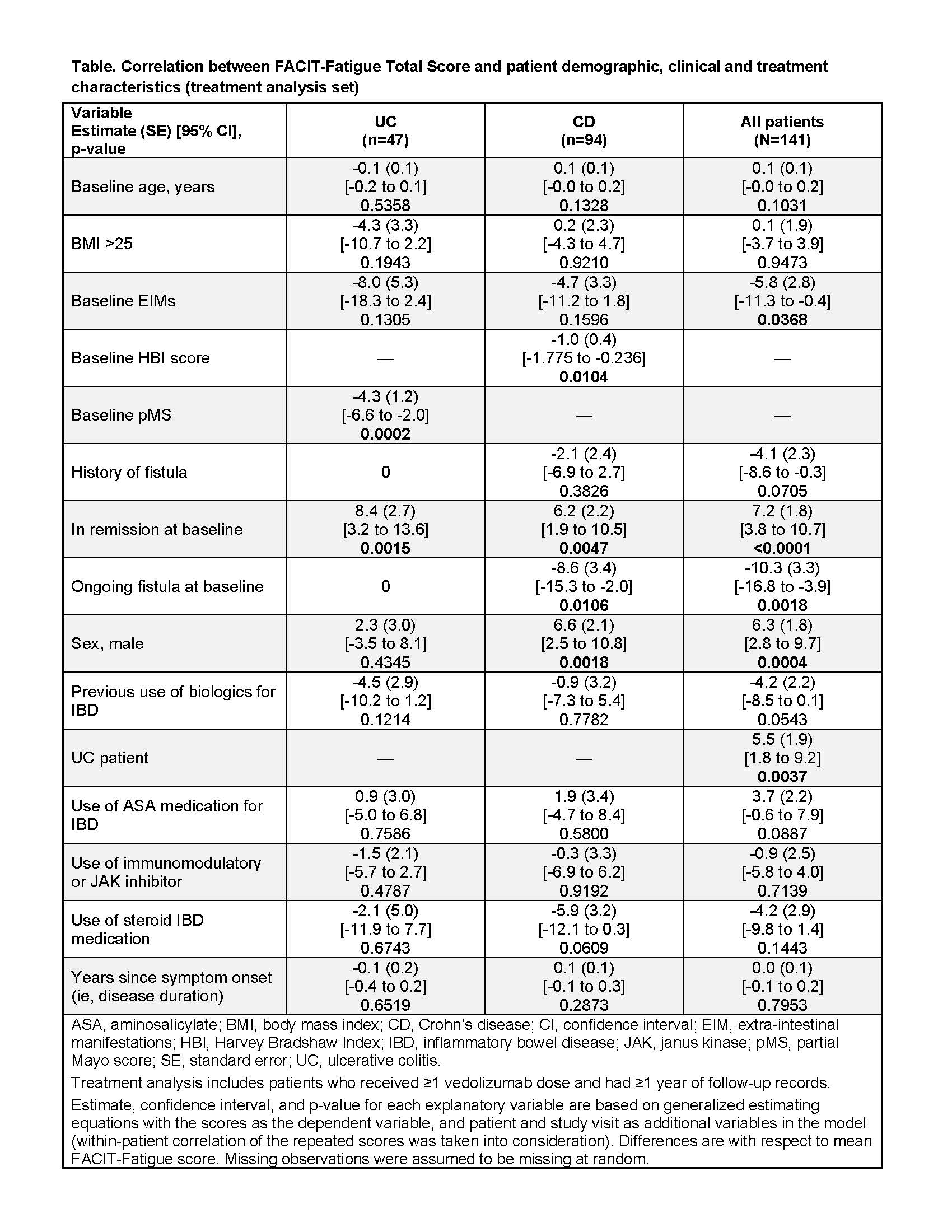
Ad-hoc analysis from the prospective observational Belgian VDZ registry (started, November 2016; data cutoff, February 2019), a sub-study of the European VDZ post-authorization safety study (ENcePP EUPAS6469) included pts aged ≥18 years with UC or CD with ongoing VDZ intravenous therapy (≥2 weeks) at recruitment. At baseline (recruitment) and every 6 months, physicians collected data (follow-up was 3 years or 1 year after last dose if VDZ was discontinued [whichever occurred first] and pts completed the 13-Item Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scale. Clinical remission was defined as Harvey-Bradshaw Index (HBI) score <5 for CD, and partial Mayo score (pMS) <2 with no individual subscore >1 for UC. This analysis explored the association (using generalized estimating equations) between all available FACIT-F total scores and baseline demographic, clinical, and treatment characteristics in the treatment analysis set (TAS; pts had ≥1 VDZ dose and ≥1 year of follow-up records).
Results
The registry enrolled 202 VDZ-treated UC and CD pts from 19 Belgian centres. TAS included 141 pts (UC 47, CD 94); median follow-up was 537 days; 140/141 pts had ≥1 FACIT-F score. In the UC and CD groups, respectively, 68% and 42% of pts were male; median (IQR) age at index date was 51 (37-59) and 40 (29-52); and baseline median (IQR) FACIT-F total score was 39 (32-46) and 32 (24-40). Lower FACIT-F score (more fatigue) was associated with higher pMS in UC (p<0.001), and higher HBI score (p=0.01) and having ongoing fistulas at baseline (p=0.01) in CD (Table). Less fatigue was associated with being in remission at baseline in both UC and CD (p<0.01). Being male was associated with less fatigue in the overall population and CD (p<0.01), but not in UC alone. Baseline occurrence of extra intestinal manifestations (EIMs) was associated with more fatigue in the overall group (p=0.037); however few pts (UC 4; CD 8) had EIMs. UC pts had less fatigue than CD pts (p=0.004). From baseline to month 24, the change in median (IQR) FACIT-F total score was -3.0 (-12.0 to 5.0) points for UC and 3.5 (-1.0 to 18.0) for CD.
Conclusion
These real-world data from a long-term registry study of VDZ-treated pts in Belgium demonstrate that higher pMS and HBI score, active fistulae, and EIM occurrence at baseline may be associated with more fatigue in IBD pts. Pts treated with VDZ had no significant change in FACIT-F total score over 24 months.