P533 Predictors of sustained remission after infliximab de-escalation in patients with inflammatory bowel diseases
Kantasiripitak, W.(1);Outtier, A.(2,3);Thomas, D.(1);Sabino, J.(2,3);Vermeire, S.(2,3);Ferrante, M.(2,3);Dreesen, E.(1);
(1)University of Leuven, Department of Pharmaceutical and Pharmacological Sciences, Leuven, Belgium;(2)University Hospitals Leuven, Department of Gastroenterology and Hepatology, Leuven, Belgium;(3)University of Leuven, Department of Chronic Diseases and Metabolism, Leuven, Belgium;
Background
Infliximab (IFX) de-escalation (i.e., extending the dosing interval and/or decreasing the dose) has been attempted in patients with inflammatory bowel disease (IBD) who sustained treatment response following an earlier IFX escalation. We aimed to identify predictors of sustained disease remission after IFX de-escalation.
Methods
Data of 30 adult patients (17 Crohn’s disease [CD], 13 ulcerative colitis [UC]), who underwent IFX de-escalation following earlier escalation, were retrospectively collected from a single-centre database search. All patients were in steroid-free clinical (two-item patient-reported outcome [PRO2] ≤1 [UC] and ≤8 [CD]) and biological (C-reactive protein <5 mg/L and faecal calprotectin <250 mg/kg) remission at IFX de-escalation. The primary endpoint was sustained steroid-free clinical and biological remission during one year after de-escalation. Predictors at baseline (i.e., demographic, clinical, and biological characteristics, the IFX trough concentration (TC), and IFX clearance (CL) [estimated using a posteriori prediction with the Dreesen model1; NONMEM v7.5]) were identified using univariate Cox regression analysis (P<0.05). Markers for steroid-free combined clinical and biological remission at the infusions during the study period (secondary endpoint) were evaluated using a multivariable generalised linear model (mGLM).
Results
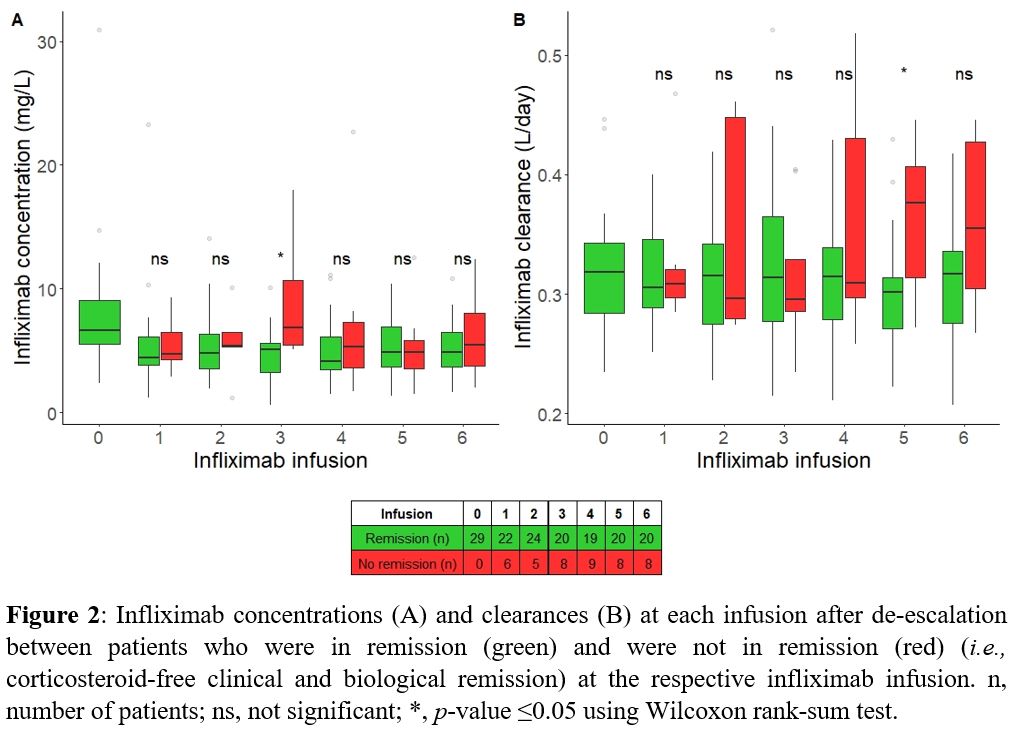
The percentage of patients who sustained steroid-free clinical and biological remission gradually decreased over time to 57% at 180 days and 37% at 360 days (Fig 1). A lower baseline PRO2 was predictive for attaining the primary endpoint (P=0.039), regardless of IBD type. One unit increase in PRO2 increased the probability of losing remission by 17% (95% CI: 0.8%-36%). The IFX TC at baseline was similar between patients with and without sustained remission during the first year (median 6.8 [IQR 6.0-7.6] mg/L and 6.0 [5.1-9.2] mg/L, respectively; P>0.05). Throughout the study period, neither IFX TCs nor IFX CLs predicted attaining the primary endpoint (P >0.05).
At the fifth infusion, patients who did not attain the secondary endpoint had a higher IFX CL (P=0.025), but the IFX TC was not different (Fig 2). In mGLM, the estimated IFX CL, but not the IFX TC itself, was identified as a predictor for attaining the secondary endpoint (P=0.016). A 0.1-unit increase in IFX CL (L/day) increased the probability of losing remission 3,227-fold.
Conclusion
Lower PRO2 at IFX de-escalation predicted a higher chance of sustained remission. Monitoring the IFX CL using a Bayesian forecasting software tool may provide more insight into disease activity than monitoring the IFX concentration as such. A prospective study is awaited to confirm our findings (MODIFI; NCT04982172).
Ref: 1 Dreesen BJCP. 2020