P534 Dual biologic or small molecules therapy in refractory paediatric inflammatory bowel disease (DOUBLE-PIBD): A multi-center study from the paediatric IBD Porto group of ESPGHAN
Cohen, S.(1);Olbjorn, C.(2);Kolho, K.L.(3); Aloi, M.(4);Musto, F.(4);de-Carpi, J.M.(5);Lozano-Ruf, A.(5);Yogev, D.(6);Matar, M.(7);Scarallo, L.(8); Bramuzzo, M.(9); de Ridder, L.(10); Kang, B.(11);Norden, C.(12);Wilson, D.C.(13);Tzivinikos, C.(14); Turner, D.(6);Yerushalmy-Feler, A.(1)*;
(1)Tel Aviv Sourasky Medical Center and the Sackler Faculty of Medicine Tel Aviv University, Pediatric Gastroenterology Institute, Tel Aviv, Israel;(2)Akershus University Hospital, Department of Paediatric and Adolescent Medicine, Lørenskog, Norway;(3)Children's Hospital and University of Helsinki- and Tampere University, Department of Paediatric Gastroenterology, Helsinki, Finland;(4)Umberto I Hospital- Sapienza University of Rome, Department of Maternal and Child Health- Pediatric Gastroenterology and Liver Unit, Rome, Italy;(5)Hospital Sant Joan de Déu, Department of Pediatric Gastroenterology- Hepatology and Nutrition, Barcelona, Spain;(6)Shaare Zedek Medical Center- the Hebrew University of Jerusalem, The Juliet Keiden Institute of Pediatric Gastroenterology and Nutrition, Jerusalem, Israel;(7)Schneider Children's Medical Center and the Sackler Faculty of Medicine- Tel Aviv University, Institute of Gastroenterology- Nutrition and Liver Diseases, Tel Aviv, Israel;(8)Meyer Children's Hospital- 50139, Gastroenterology and Nutrition Unit, Florence, Italy;(9)Institute for Maternal and Child Health-IRCCS "Burlo Garofolo", Gastroenterology- Digestive Endoscopy and Nutrition Unit, Trieste, Italy;(10)Erasmus Medical Center/Sophia Children's Hospital, Department of Paediatric Gastroenterology, Rotterdam, The Netherlands;(11)School of Medicine- Kyungpook National University- 130 Dongdeok-ro- Jung-gu, Department of Pediatrics, Daegu 41944, Korea- Republic Of;(12)Copenhagen University Hospital, The Paediatric Department, Hvidovre, Denmark;(13)Royal Hospital for Sick Children, Department of Paediatric Gastroenterology and Nutrition, Edinburgh, United Kingdom;(14)Al Jalila Children's Specialty Hospital, Pediatric Gastroenterology, Dubai, United Arab Emirates;
Background
Current data on dual biologic or small molecules therapy in children are limited. The aim of the study was to evaluate the effectiveness and safety of dual therapy in paediatric patients with IBD.
Methods
This was a retrospective multicenter study from 14 centers affiliated with the IBD interest and Porto groups of ESPGHAN. We included children with IBD who were treated with combination of biologic agents or biologic and small molecule, with at least 3 months of follow-up under this therapy. Demographic, clinical, laboratory, endoscopic and imaging data were collected. Adverse events were recorded. All analyses were done in the intention-to-treat population. Children that discontinued therapy were considered treatment failures and were imputed for non-response, while missing data was imputed according to the last observation carried forward method.
Results
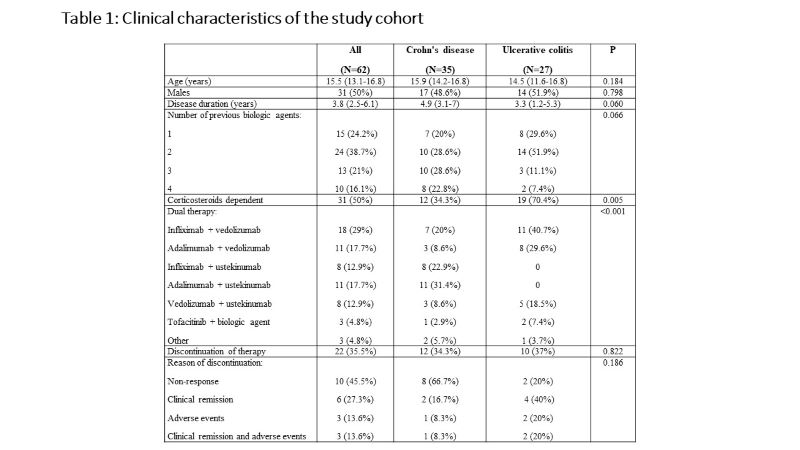
Sixty-two children [35 Crohn's disease (CD), 27 ulcerative colitis (UC)], with a median age of 15.5 (IQR 13.1-16.8) years and disease duration of 3.8 (IQR 2.5-6.1) years were included. All children failed previous biologics and 47 (76%) failed at least two biologic agents. The dual therapy included anti-TNF agent and vedolizumab in 30 children (48%), anti-TNF and ustekinumab in 21 children (34%), vedolizumab and ustekinumab in 8 children (13%), and tofacitinib and other biologics in 3 children (5%). Clinical remission was observed in 21 (35%), 30 (50%) and 38 (63%) children at 3, 6 and 12 months, respectively. Of 27 children who were treated with corticosteroids at baseline, 20 (74.1%) were weaned within 3 months after the initiation of dual therapy. At 12 months of follow up, normalization of C-reactive protein and decrease in fecal calprotectin to < 250 mcg/g were achieved in 75% and 64%, respectively. Endoscopic and transmural healing were observed in 2/23 (9%) and 5/16 (31%) children, respectively. Male sex and diagnosis of UC were associated with higher likelihood of clinical remission (p=0.017 and p=0.020, respectively). Adverse events were reported in 29 (47%) children. While most adverse event were mild, 8 were regarded as serious and 6 (10%) led to discontinuation of dual therapy. The serious adverse events included infusion reaction to infliximab, fatigue and headache following vedolizumab infusion, severe skin eruptions (3 patients), cellulitis and skin abscess, elevated liver enzymes and deep vein thrombosis.
Conclusion
Dual biologic or small molecules therapy may be effective in children with otherwise refractory IBD. The risk of serious adverse events should be considered before recommending dual therapy.