P534 Qualitative perspectives of parent and child participants from a trial of faecal microbiota transplant for paediatric ulcerative colitis
J. Popov1,2, E. Hartung1, L. Hill1,3, U. Chauhan4, N. Pai1,5, McMaster Paediatric FMT Research Program
1Division of Gastroenterology and Nutrition, Department of Paediatrics, McMaster University, Hamilton, Canada, 2College of Medicine and Health, University College Cork, Cork, Ireland, 3Department of Exercise Science and Sports Medicine, University of Cape Town, Cape Town, South Africa, 4Division of Gastroenterology and Hepatology, Department of Medicine, McMaster University, Hamilton, Canada, 5Department of Medicine, Farncombe Family Digestive Health Research Institute, McMaster University, Hamilton, Canada
Background
Faecal microbiota transplant (FMT) is being studied across a range of therapeutic indications including ulcerative colitis (UC). Pediatric patients may have unique perspectives on microbiome-based therapeutics given their younger age, fewer comorbidities, and greater susceptibility to microbial influences. We recently conducted the first randomised-controlled trial (RCT) of FMT for pediatric UC (PediFETCh Trial) and conducted qualitative interviews of child participants and their parents. This study aims to describe the experiences and perceptions of these children who received FMT, and their parents.
Methods
Participants in the PediFETCh Trial (ClinicalTrials.gov: NCT02487238) were invited to participate in face-to-face, semi-structured interviews. Interviews were audiotaped, transcribed, and analyzed using open coding (NVivo 12 Pro).
Results
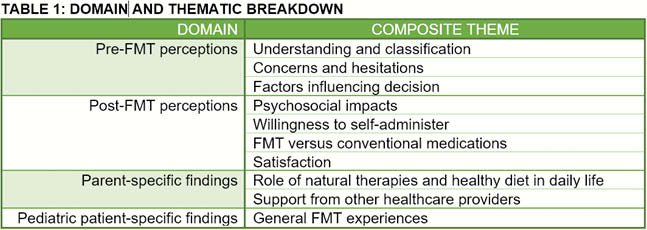
8 pediatric participants and 8 parents were interviewed. Data were summarised across 4 domains and 11 composite themes (Table 1). Most children and parents saw FMT as a ‘safe, natural’ treatment. Prior to enrollment, children were concerned about receiving ‘someone else’s poo’ and potential physical discomfort, while parents were concerned about transmission of enteric infections and psychiatric diseases. Both groups felt their decision to pursue FMT was influenced by frustration with lack of response to medications, and fear of medication-related side effects. Following completion of the study, most children and parents had no concerns about potential side effects of FMT, and children reported feeling ‘completely normal’. Children were split between preferring FMT or medication therapies. The convenience of medications was valued, while others favoured FMT for its symptomatic improvement and perceived naturality. It was noted that some alternative healthcare practitioners did not support patients’ desire to pursue FMT.
Conclusion
Our study offers valuable insight into the experiences of receiving FMT in pediatric participants and their parents. This data suggest a high rate of acceptance and interest in FMT-based therapeutics in this population and offers strategies to improve the delivery of FMT in future pediatric-focused RCTs.


