P537 Real-world long-term effectiveness of ustekinumab in Crohn’s disease: Results from the ENEIDA registry
M.I. Iborra Colomino1, B. Beltrán1, A. Fernández-Clotet2, E. Iglesias Flores3, P. Navarro4, M. Rivero5, A. Gutiérrez6, M. Sierra-Ausin7, F. Mesonero8, R. Ferreiro-Iglesias9, J. Hinojosa10, X. Calvet11, B. Sicilia12, C. González-Muñoza13, B. Antolín14, M. González Vivo15, A.Y. Carbajo16, S. García17, A. Martín-Cardona18, G. Surís Marín19, M.D. Martín-Arranz20, R. De Francisco21, F. Cañete22, T. Carlos23, F. Gomollón24, R. Lorente25, I. Rodríguez-Lago26, A. Forés-Bosch27, E. Bernardos28, L. Ramos29, P. Delgado30, A. Hernández31, M. Van Domselaar32, D. Hervás33, E. Domènech34, P. Nos1
1Department of Gastroenterology, Hospital Universitario y Politécnico La Fe, Valencia, Spain, 2Department of Gastroenterology, Hospital Clínic Barcelona, Barcelona, Spain, 3Department of Gastroenterology, Hospital Universitario Reina Sofía de Córdoba, Córdoba, Spain, 4Department of Gastroenterology, Hospital Clínico Universitario de Valencia, Valencia, Spain, 5Department of Gastroenterology, Hospital Universitario Marqués de Valdecilla, Santander, Spain, 6Department of Gastroenterology, Hospital General de Alicante, Alicante, Spain, 7Department of Gastroenterology, Complejo Asistencial Universitario de León, León, Spain, 8Department of Gastroenterology, Hospital Ramón y Cajal Madrid, Madrid, Spain, 9Department of Gastroenterology, Hospital Universitario de Santiago, Santiago de Compostela, Spain, 10Department of Gastroenterology, Hospital de Manises, Valencia, Spain, 11Department of Gastroenterology, Corporació Sanitària Parc Taulí de Sabadell, Sabadell, Spain, 12Department of Gastroenterology, Hospital Universitario de Burgos, Burgos, Spain, 13Department of Gastroenterology, Hospital Santa Creu i Sant Pau, Barcelona, Spain, 14Department of Gastroenterology, Hospital Clínico Universitario de Valladolid, Valladolid, Spain, 15This email address is being protected from spambots. You need JavaScript enabled to view it., Hospital Parc de Salut Mar, Barcelona, Spain, 16Department of Gastroenterology, Hospital Universitario Río Hortega, Valladolid, Spain, 17Department of Gastroenterology, Hospital Miguel Servet, Zaragoza, Spain, 18Department of Gastroenterology, Hospital Mutua de Terrassa, Terrassa, Spain, 19Department of Gastroenterology, Hospital de Bellvitge, Barcelona, Spain, 20Department of Gastroenterology, Hospital Universitario La Paz, Madrid, Spain, 21Department of Gastroenterology, Hospital Central de Oviedo, Oviedo, Spain, 22Department of Gastroenterology, Hospital Germans Trias i Pujol, Barcelona, Spain, 23Department of Gastroenterology, Hospital Clínico San Carlos, Madrid, Spain, 24Department of Gastroenterology, Hospital Clínico Universitario Lozano Blesa, Zaragoza, Spain, 25Department of Gastroenterology, Hospital General Universitario de Ciudad Real, Ciudad Real, Spain, 26Department of Gastroenterology, Hospital Universitario de Galdakao, Galdakao, Spain, 27Department of Gastroenterology, Hospital General de Castellón, Castellón, Spain, 28Department of Gastroenterology, Hospital General La Mancha Centro, Alcázar de San Juan, Spain, 29Department of Gastroenterology, Hospital Universitario de Canarias, Santa Cruz de Tenerife, Spain, 30Department of Gastroenterology, Hospital General de Granollers, Granollers, Spain, 31Department of Gastroenterology, Hospital Universitario Nuestra Señora de la Candelaria, Santa Cruz de Tenerife, Spain, 32Department of Gastroenterology, Hospital Universitario de Torrejón, Madrid, Spain, 33Department of Biostatistics, Hospital Universitario y Politécnico La Fe, Valencia, Spain, 34Department of Gastroenterology, Hospital Universitario Germans Trias i Pujol, Badalona, Spain
Background
There are limited data of long-term ustekinumab administered according to the doses recommended in the UNITI studies. The objective of this study was to assess the real‐world, long‐term effectiveness of ustekinumab in refractory Crohn’s disease (CD) (LONG-CROHNUSK Study).
Methods
Multicentre study of CD patients starting ustekinumab at the recommended dose based on weight ~6 mg/kg IV week 0, 90 mg SC week 8 and maintenance 90 mg SC every 8 or 12 weeks and with 1 year of follow-up. Values for Harvey‐Bradshaw Index (HBI), endoscopic activity, C reactive protein (CRP) and faecal calprotectin (FC) were recorded at baseline and at weeks 26 and 52. Demographic and clinical data, previous treatments, adverse events (AEs), surgeries and hospitalisations were documented. Potential predictors of clinical and endoscopic remission were examined.
Results
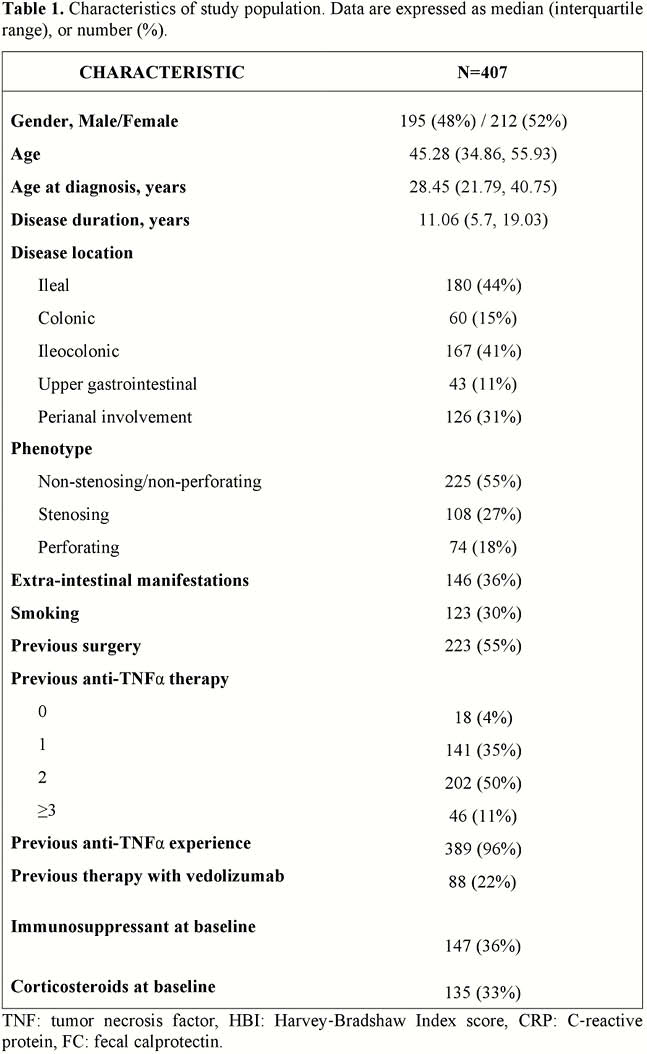
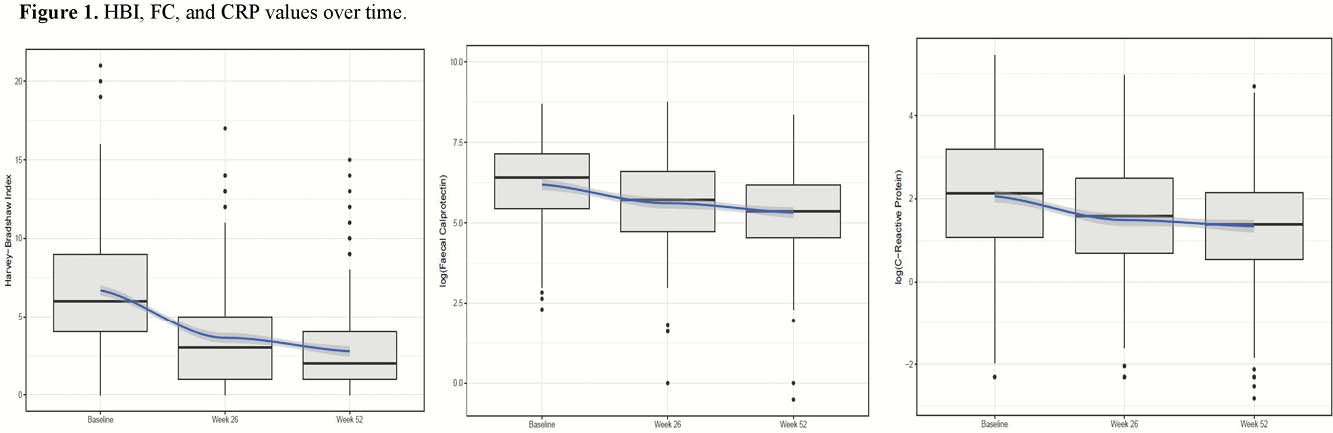
Four hundred and seven patients were analysed (Table 1). For the maintenance dose, ustekinumab 90 mg was administered SC every 12, 8 and 4 weeks in 56 (14%), 318 (84.5%) and 7 (1.5%) patients, respectively. An interval reduction was applied for 118 patients (29%). Before 52 weeks, treatment discontinuation occurred in 71 patients (17%). At baseline, 295 (72%) had an HBI >4 points. Of these, 169 (57%) and 190 (64%) achieved clinical remission at weeks 26 and 52, respectively. FC levels returned to normal (<250 μg/g) in the 44% and 54% of the patients at weeks 26 and 52, respectively. CRP returned to normal (<3 mg/l) in 36% and 37% of the patients at weeks 26 and 52 respectively. HBI, FC, and CRP values over time are shown in Figure 1. Of the 159 patients with endoscopy at 52 weeks, 25 (16%) were in remission and 58 (36%) presented mild activity. Thirty-eight (9.3%) patients worsened extra-intestinal manifestations and 33 (8%) their perianal disease. AEs were recorded in 54 patients, 73 were hospitalised and 53 had surgery. An association was shown for fewer previous anti‐TNF agents and ileal localisation with clinical remission, and for endoscopic severity at baseline with poor response. No factors correlated with endoscopic remission.
Conclusion
This is the first study to show the real‐world long-term effectiveness, endoscopic improvement and safety of ustekinumab administered according to the recommended induction regimen in a cohort of highly refractory CD patients.


