P540 Real-world evidence on treatment switching in patients with moderate-to-severe ulcerative colitis: a systematic review of literature
Singh, H.(1);Wilson, L.(2);Pandey, A.(3);Tencer, T.(4);Kumar, J.(4);
(1)Amaris Consulting Ltd, Health Economics & Market Access HEMA, Toronto, Canada;(2)Amaris Consulting Ltd, Health Economics & Market Access HEMA, Shanghai, China;(3)Amaris Consulting Ltd, Health Economics & Market Access HEMA, London, United Kingdom;(4)Bristol Myers Squibb, Global HEOR, Princeton, United States
Background
Current approved therapies for the treatment of moderate-to-severe ulcerative colitis (UC) have well-established efficacy; however, some patients may fail to respond or may lose their response over time, resulting in dose escalation or treatment switching. The objective of this systematic literature review was to summarize the real-world evidence on treatment switching in UC and the associated clinical, safety and economic outcomes.
Methods
A literature search of Embase and MEDLINE (from database inception to August 2020) and conference proceedings (2017 to 2020) was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. The population of interest included adult patients (≥18 years) with UC who experienced treatment switching on anti-tumour necrosis factor alpha agents (anti- TNFα) - (adalimumab [ADA], infliximab [IFX], golimumab [GOL]), vedolizumab (VDZ), tofacitinib (TOFA), and ustekinumab (USTE). Outcomes of interest included all available clinical, safety and economic outcomes.
Results
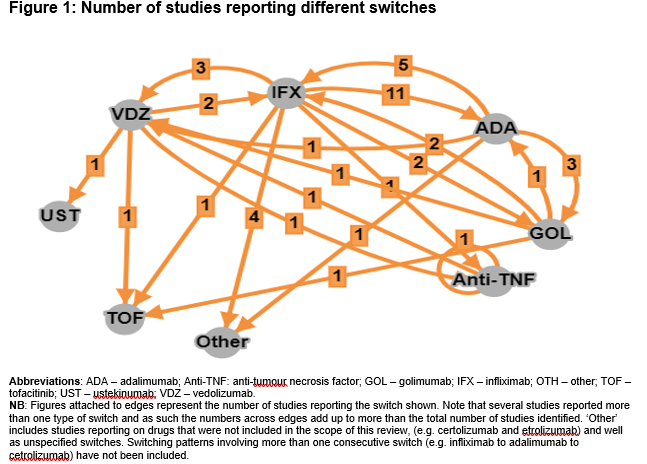
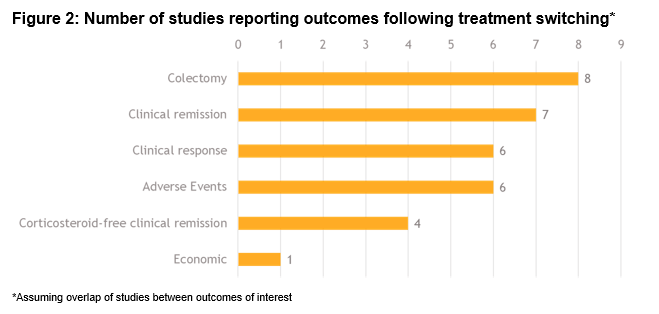
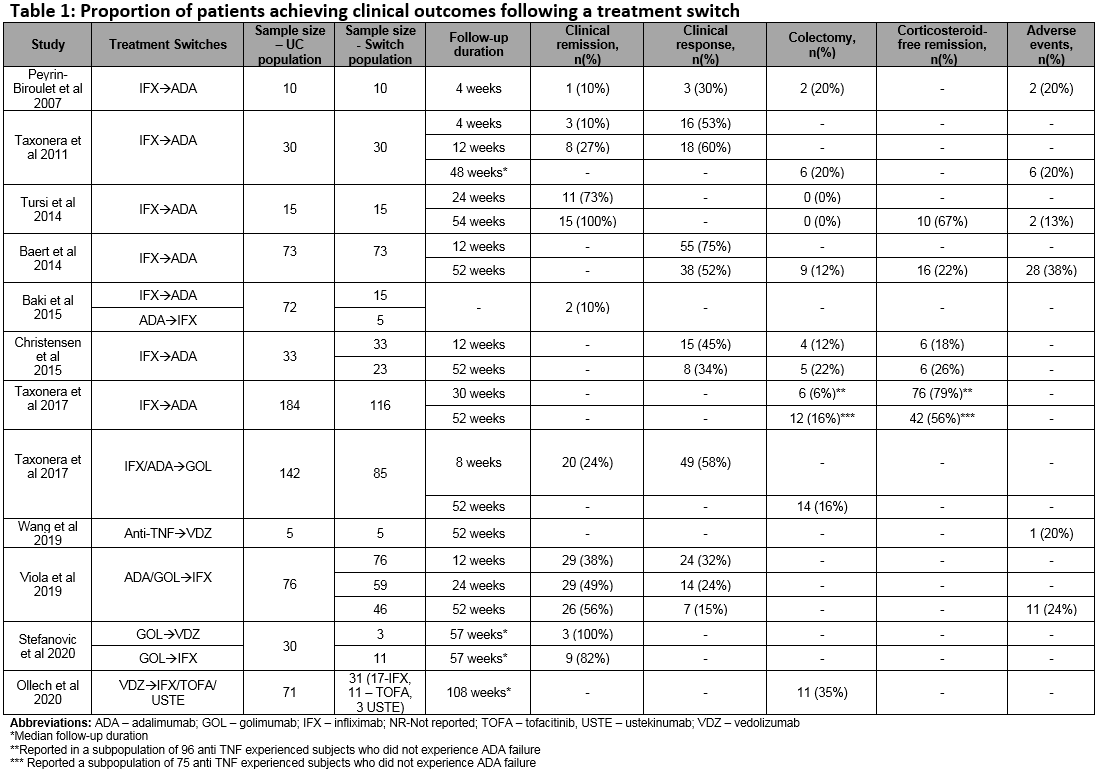
Of a total 1593 studies screened, 29 met the eligibility criteria. Of these, 22 (76%) reported switching rates of 4-63% (sample size: 4-1274). A total of 7 studies reported outcomes in populations comprised entirely of switched patients (sample size: 5-76). Figure 1 includes the directed connectivity graph showing the number of studies reporting different switches. IFX to ADA was the most frequently reported switch (n=11 studies;35%). Figure 2 and Table 1 include the number of studies and proportion of patients reporting outcomes following a treatment switch, respectively. Heterogeneity was observed in patient populations, follow-up, and definitions of response/remission. In the short term (4-12 weeks), remission rates of 10-38% have been reported that increased to 49-100% in the long-term (24-57 weeks). In contrast, the clinical response rates decreased over time (30-58% over 4-12 weeks versus 15-56% over 24-57 weeks). Colectomy and corticosteroid-free remission were reported in 0-35% and 18-79% of patients, respectively. Rate of adverse events varied from 13–38%, with a switch to IFX from other anti-TNFs associated with the highest rate. One study reported higher mean quarterly total healthcare costs in patients who switched within anti-TNFs (IFX>ADA, ADA>IFX) compared to those who did not ($15,004 vs $9632).
Conclusion
Treatment switching in the management of moderate-to-severe UC is common in routine clinical practice and is associated with suboptimal outcomes and potentially increased costs. There remains a need for additional treatment options that provide long-term durable disease control.


