P541 Effectiveness, safety and durability of vedolizumab in active moderate-severe Crohn's Disease
Morales Bermúdez, A.I.(1);Bracho González, M.(1);Gómez Rodríguez, P.(1);Olmedo Martín, R.(1);
(1)Regional University Hospital of Málaga, Digestive Diseases Department, Málaga, Spain;
Background
Vedolizumab (VDZ) has demonstrated efficacy and safety in moderate-severe Crohn's Disease (CD) in GEMINI registration trials. Nevertheless, the patient profile in actual clinical practice differs from that included in controlled studies so additional data are needed. The primary objective of our study was to evaluate the effectiveness, safety and durability of VDZ in active moderate-severe CD in real clinical practice.
Methods
Unicentric retrospective observational study. All patients treated in our center with VDZ with moderate-severe CD were included. Effectiveness was assessed 16 weeks after treatment iniciation and at the last visit with available data. Remission (RmLE) and steroid-free response (RsLE) were considered to be the achievement of a Harvey-Bradshaw index (HBI) ≤4 or a decrease of at least 3 points from the previous HBI. To evaluate the durability of VDZ, a Kaplan-Meier survival curve was performed. In addition, adverse effects attributable to VDZ during follow-up were recorded.
Results
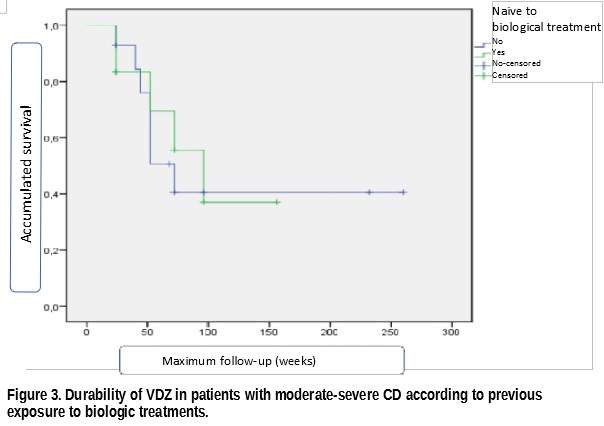
We included 26 patients whose clinical-demographic features are shown in Table 1. The median follow-up of patients was 52 weeks (IQR= 24-78). At 16 weeks after initiation of VDZ treatment, RmLE and RsLE were achieved in 19.2% and 69.2% of patients respectively. At the last follow-up visit, 27% of patients achieved RmLE with 61.5% presenting RsLE (Figure 1). The probability of retention of VDZ treatment at median follow-up was 57%, not differing significantly between bionaive patients from those with previous failure to other biologic treatments (Figures 2 and 3). There were no serious adverse effects or effects that required discontinuation of VDZ. Three mild adverse effects were documented in 11.5% of patients (arthralgia, dyspnea and a mild viral infection).



Conclusion
In our series of patients, VDZ proved effectiveness and safety in line with that described in other real clinical practice series. The durability of VDZ at one year in this series of refractory CD was also considerable and did not vary according to previous exposure to biologic agents.


