P542 The effect of age on infliximab pharmacokinetics in patients with inflammatory bowel disease
W. Kantasiripitak1, B. Verstockt2,3, T. Lobatón2,3,4, D. Thomas1, A. Gils1, S. Vermeire2,3, M. Ferrante2,3, E. Dreesen1
1Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, Leuven, Belgium, 2Department of Gastroenterology and Hepatology, University Hospitals Leuven, Leuven, Belgium, 3Department of Chronic Diseases Metabolism and Ageing, KU Leuven, Leuven, Belgium, 4Department of Gastroenterology, Ghent University Hospital, Ghent, Belgium
Background
Data remain scarce in terms of efficacy and safety of infliximab (IFX) treatment in elderly patients with inflammatory bowel disease.1 Our aim was to employ a population pharmacokinetic (popPK) model to improve our understanding of factors impacting variability in IFX exposure in elderly patients with ulcerative colitis (UC) and Crohn’s disease (CD).
Methods
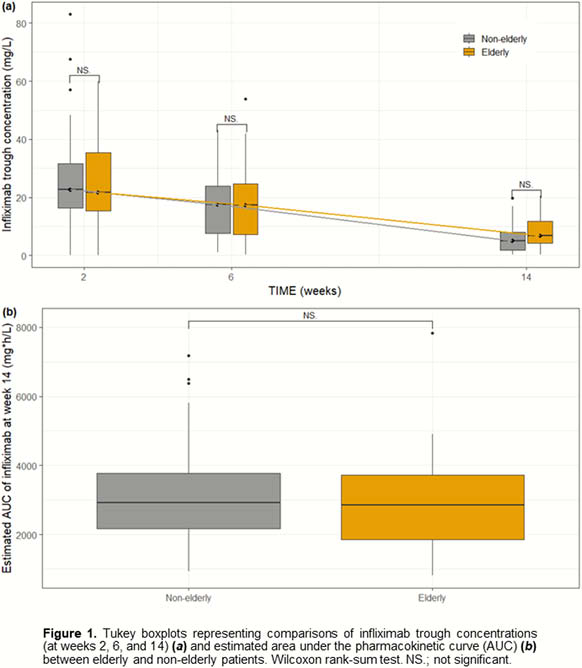
IFX concentration–time data during induction therapy (week 2, 6 and 14) of 104 patients were obtained from a retrospective case–control study2 (
Results
Median age was 62 years (IQR 38–68). A total of 46 of 104 patients (44%) were elderly. A one-compartment model with linear clearance showed adequate descriptive and predictive ability. The estimated popPK parameters (typical value [%RSE]) were clearance CL (0.346 l/day [4%]) and volume of distribution V (6.42 l [5%]). The elimination half-life of IFX in the elderly was not significantly different from the non-elderly (12.6 days vs. 11.4 days,
Conclusion
Older age is an independent predictor of lower clearance. However, the effect of age did not pronounce on infliximab exposure in our elderly patient cohort as a result of confounding effects of fat-free mass and serum albumin.
LeBlanc
Lobatón
Paul


