P549 A virtual clinic enhances the quality use of anti-TNF dose intensification and rates of treatment success using a treat-to-target approach compared with standard outpatient care in Crohn’s disease
A. Srinivasan1,2,3, D.R. van Langenberg1,3, R. Little4, M.P. Sparrow3,4, P. De Cruz2,5, M.G. Ward3,4
1Department of Gastroenterology, Eastern Health, Melbourne, Australia, 2Department of Gastroenterology, Austin Health, Melbourne, Australia, 3Department of Medicine, Monash University, Melbourne, Australia, 4Department of Gastroenterology, Alfred Health, Melbourne, Australia, 5Department of Medicine, University of Melbourne, Melbourne, Australia
Background
Virtual clinics (VC) represent a novel model-of-care which promote quality use of biologics and facilitate a treat-to-target (T2T) approach. We aimed to evaluate the clinical and process-driven outcomes of intensified anti-TNF therapy for secondary loss of response (SLoR) in a VC compared with standard outpatient care (SoC).
Methods
A retrospective multicenter, parallel observational cohort study of patients with Crohn’s disease (CD) who underwent anti-TNF dose intensification to address SLoR with up to 2-years of follow-up was undertaken. Objective assessments of disease activity and anti-TNF trough levels at SLoR then during subsequent 6-month semesters were compared longitudinally between VC and SoC groups. ‘Anti-TNF escalation success’ was the primary endpoint, defined as achieved when two consecutive 6-month semesters of objective assessment demonstrated inactive disease. Dose intensification was defined as ‘appropriate’ if there was both objective evidence of disease activity
Results
149 patients (69 VC vs. 80 SoC) underwent infliximab (
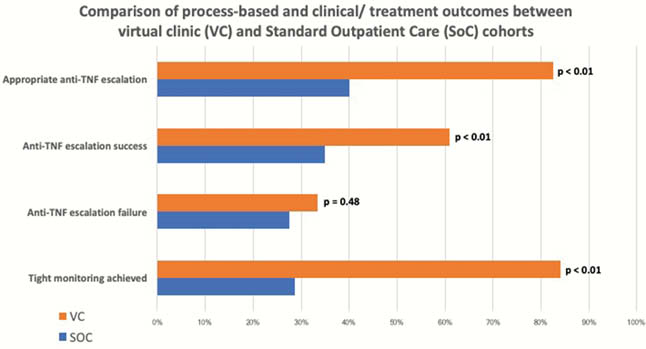
Conclusion
This study favoured a VC-led model of care over standard outpatient-based IBD care in facilitating an effective T2T approach to CD management. A VC model of care improved quality use of intensified anti-TNF therapy through processes that promoted appropriate dose intensification and encouraged more frequent anti-TNF dose de-escalation. Moreover, tight semester-based monitoring using a T2T-based strategy facilitated by the VC was associated with improved treatment outcomes.


