P552 Higher Serum Ustekinumab Levels Correlate With Higher Rates Of Steroid-Free Deep Remission And Endoscopic Healing In Patients With Inflammatory Bowel Diseases
Yarur, A.(1);Ungaro, R.(2);Jain, A.(3);Nunex, L.(1);Spencer, E.(2);Bruss, A.(1);Berens, B.(1);Beniwal-Patel, P.(1);Dervieux, T.(3);Dubinsky, M.(2);
(1)Medical College of Wisconsin, Department of Gastroenterology, Milwaukee, United States;(2)The Mount Sinai Hospital, Medicine, New York, United States;(3)Prometheus Labs, Research & Developement, San Diego, United States;
Background
Ustekinumab (UST) is currently approved for the treatment of Crohn’s disease (CD) and ulcerative colitis (UC). However, a significant number of patients either partially respond or do not benefit at all. With other biologics, this non-response has partially been explained by sub-optimal pharmacokinetics. The aim of this study was to assess the association between UST trough levels (UTL), antibodies to UST (ATU) and clinical/endoscopic disease activity.
Methods
In this prospective cross-sectional study, we enrolled patients with CD or UC on maintenance doses of UST (≥6 months). We collected clinical data, UTL and ATU titers using a drug-tolerant assay, biomarker levels (c-reactive protein [CRP] and fecal calprotectin [FC]), Harvey Bradshaw Index (HBI) and simple endoscopic score (SES-CD) in CD, partial and endoscopic Mayo score (PMS and EMS) in UC. The primary outcome was deep steroid-free remission (SFDR) defined as HBI <5 in CD and PMS<2 in UC and a normal CRP/FC while off corticosteroids (prednisone or budesonide). The secondary outcome was endoscopic healing defined as an EMS≤1 in UC or SES-CD≤2.
Results
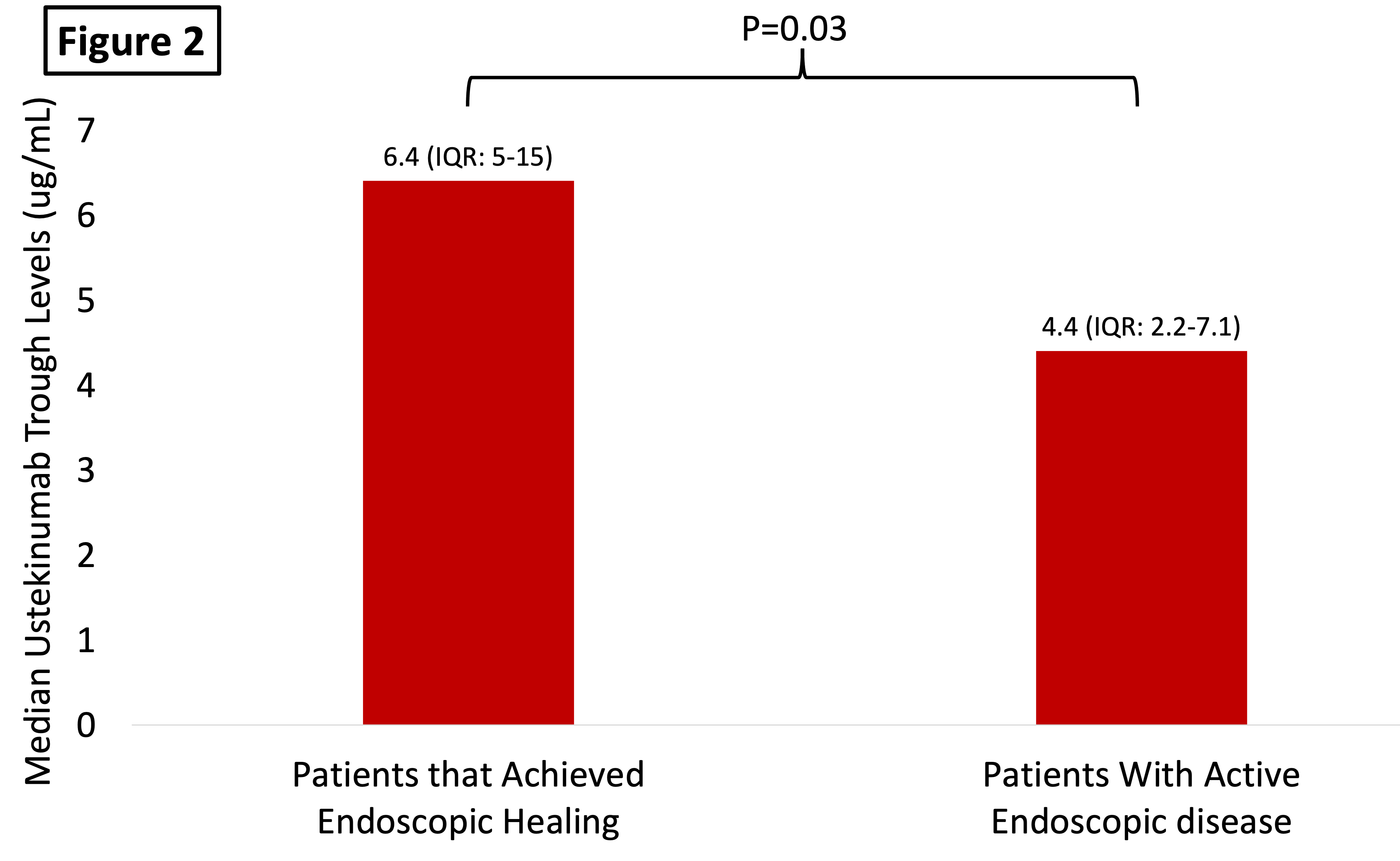
136 patients were included; 123 (90%) had CD, mean age was 43 years (SD:17), and 73 (54% were women). The median time on UST was 40 weeks (IQR: 32-78). Median UTL were 5.5 µg/mL (IQR: 3-8.8 µg/mL) and no patients had detectable ATU. There was a positive correlation between UTL and albumin (rho:0.35 [p<0.01]) and a negative correlation between UTL with body mass and CRP (rho:-0.32 [p=0.005] and rho: -0.32 [0.0002], respectively). Among the study population, 48/136 (35.2%) was in SFDR and 26 (42%) were in endoscopic remission. Patients in SFDR and in endoscopic remission had significantly higher UTL vs. that did not (6.4 µg/mL [4.6-11.7] vs. 4.8 µg/mL [2.5-7.6]; p<0.01 and 6.4 µg/mL [5-15] vs. 4.4 µg/mL [2.2-7.1], p=0.03 respectively) – Figure 1 and 2. There were no differences in UTL between those patients on monotherapy and those on combination therapy with a thiopurine or methotrexate (5.8 µg/mL [3.4-9.3] vs. 4.5 µg/mL [2.3-7.9], p=0.07). Patients with a UST ≥5.45 µg/mL had a two-fold higher chance of being in steroid-free deep remission vs. those that did not (OR:2.2 [95%CI:1.1-4.5], p=0.031). When adjusting for albumin (a surrogate marker of drug clearance), higher UTL were associated with higher rates of SFDR (OR per 1 µg/mL: 1.12 [95%CI: 1.01-1.26], p=0.034).
Conclusion
Among patients with CD and UC, higher UTL were associated with better clinical and endoscopic outcomes. No patients in the cohort had developed immunogenicity. Dose-escalation, interventional studies looking into how a higher exposure-response to UST may improve rates of non-response are warranted.


