P553 Active tuberculosis and opportunistic infections: Pooled safety analysis of ustekinumab through up to 5 years across all approved indications
Ghosh, S.(1)*;Long, M.(2);Ott, E.(3);Gasink, C.(3);Baker, T.(4);Godwin, B.(3);Miao, Y.(4);Loftus, E.(5);
(1)University College Cork, College of Medicine and Health, Cork, Ireland;(2)University of North Carolina at Chapel Hill, Division of Gastroenterology and Hepatology, Chapel Hill, United States;(3)Janssen, Scientific Affairs, Horsham, United States;(4)Janssen, Research & Development, Spring House, United States;(5)Mayo Clinic College of Medicine and Science, Division of Gastroenterology and Hepatology, Rochester, United States;
Background
Ustekinumab (UST) is an approved treatment for adults with inflammatory bowel disease (IBD: Crohn’s disease [CD] and ulcerative colitis [UC]), psoriasis (PsO), and psoriatic arthritis (PsA). Here, we present pooled safety analyses in these approved indications of patients (pts) with active tuberculosis (TB) and opportunistic infections (OIs) through 5 years (yrs) of UST treatment.
Methods
Pooled data included 13 Phase 2/3 UST studies through 5 yrs of CD and PsO, 2 yrs of UC, and 1 yr of PsA. OIs were identified by clinician review. Herpes zoster (HZ) was evaluated separately. Event rates per 100 pt yrs (PYs) are presented. Concomitant immunomodulators/corticosteroids were permitted in IBD and PsA pts. All pts who received ≥1 UST dose were included. In IBD, placebo (PBO) pts included data up to the first UST dose for pts initially treated with PBO, or >16 weeks after the last UST dose for UST pts who switched to PBO.
Results
Across all approved indications, 19 OIs including TB were reported, with rates in PBO of 0.40 and UST of 0.10 through 5 yrs in 13807 PYs of follow-up (Table 1); rates of HZ were 1.21 and 0.63, respectively. Of 19 OIs, 18 were in IBD pts and 1 in a PsO pt. Overall, 14/16 pts (12/13 UST) with OIs excluding TB were also receiving confounding concomitant medications. A total of 3 active TB cases (2 pts with CD and 1 pt with UC) were reported in PBO (n=2; 1 in a Hungarian CD pt 10 months after receiving last UST dose) and UST (n=1) pts (Table 1). One active TB case was reported in an asymptomatic South African CD pt treated with UST who had a positive QuantiFERON®-TB Gold test on routine screening and bronchial brushings positive for M. tuberculosis. Both CD pts completed TB treatment with disease resolution. The most common OIs were esophageal candidiasis (UST n=3; PBO n=2) and cytomegalovirus colitis (UST n=3; PBO n=1).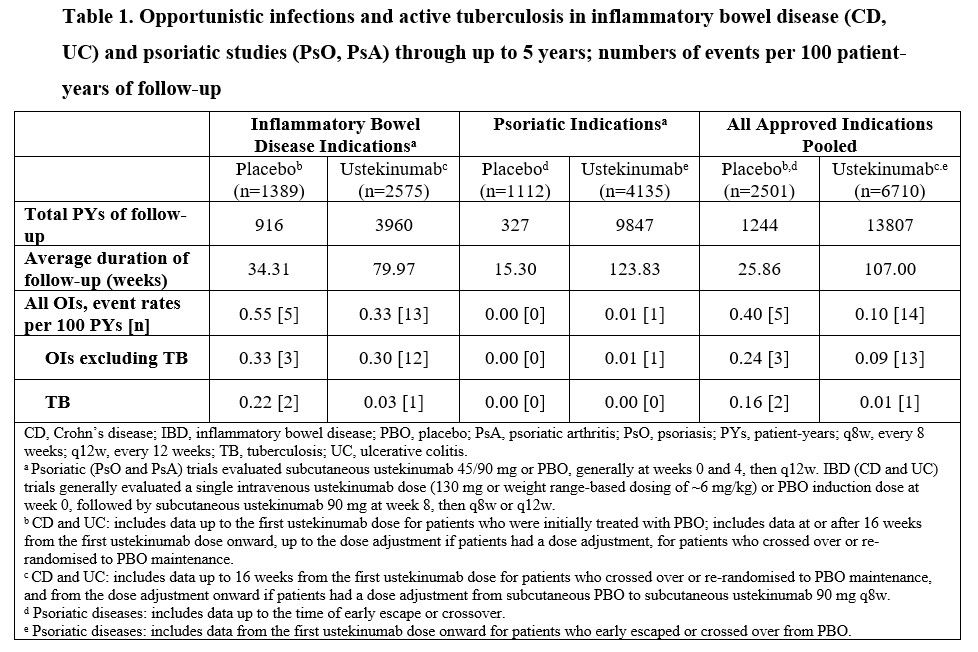
Conclusion
Rates of OIs, including active TB, in UST-treated pts were low across approved indications through up to 5 yrs with 13807 PYs of follow-up and not higher in UST pts vs PBO pts, suggesting no increased risk of OI with long-term UST treatment.


