P561 Clinical utility of therapeutic drug monitoring of adalimumab using a point of care test
Stevens, T.(1);Teichert, C.(1);Volkers, A.(1);Mostafavi Montazeri, N.(1);Bahur, B.(2);Bray, K.(3);D'Haens, G.(1);
(1)Amsterdam UMC, Gastroenterology and Hepatology, Amsterdam, The Netherlands;(2)ProciseDx Inc., Clinical Operations, San Diego, United States;(3)ProciseDx Inc., Clinical Development and Medical Affairs, San Diego, United States;
Background
Adalimumab (ADL) is a fully human monoclonal antibody against tumor necrosis factor that is approved for the management of inflammatory bowel disease (IBD). Therapeutic drug monitoring (TDM) of ADL is widely used to ensure adequate blood levels for maintenance of the clinical benefit. This study examined the clinical utility of a point of care (POC) ADL assay to facilitate TDM.
Methods
A fast (<3 min), time-resolved fluorescence resonance energy transfer (FRET) based immunoassay (Procise ADL™) was developed for measuring of ADL and its biosimilars for TDM using 20 µL of finger prick capillary whole blood or serum. To assess its clinical performance we conducted an observational study using stored frozen serum specimens from a nested cohort in a registry population collected over 24 months. Serum samples were thawed and ADL concentrations were measured with Procise ADL™. Adult patients with an established diagnosis of Crohn’s disease (CD) or ulcerative colitis (UC) who received maintenance ADL treatment were included. Primary endpoint was loss of response (LOR) defined as any of the following: (i) disease flare defined by documented worsening symptoms and abnormal endoscopy/imaging/biomarker findings leading to discontinuation of ADL; (ii) disease activity leading to change in IBD medication; (iii) increase in fecal calprotectin ≥150 mg/Gr; (iv) IBD surgery or (v) new or recurring actively draining fistula. To be evaluable LOR patients were required to have a study specimen < 60 days prior to the LOR event. Receiver-operating characteristic (ROC) curves were generated to identify optimal cut-off values to discriminate between patients with maintained response vs. LOR.
Results
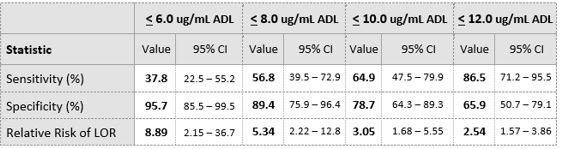
A total of 84 IBD patients (LOR=37, No LOR= 47) were included in this study. Median ADL trough levels were lower in patients who experienced loss of response compared to patients who did not (median ADL 6.0 μg/mL vs 13. μg/mL, P < 0.001, figure 1). Area-Under-the-ROC Curve (AUC) value for loss of response was 0.822 (figure 2). Optimal ADL trough cut-off value was 8 μg/mL (Table 1).
Figure 1. shows patients losing response to ADL had serum levels significantly lower than those maintaining response
Figure 2. shows ROC curve analysis of the Procise ADL test for the detection of LOR
Table 1. Showsclinical performance of Procise ADL for the detection of LOR at various ADL concentration
Conclusion
IBD patients in disease remission on maintenance ADL therapy with ADL levels below a cut-off 8.0 μg/mL had a 5.34 fold increased risk of LOR compared to those above 8.0 μg/mL . Identifying patients at high risk of LOR with a convenient POC format test enhances the clinical utility of TDM by enabling faster treatment adjustment.