P565 Efficacy and safety of long-term treatment with ustekinumab in moderate–severe ulcerative colitis patients with delayed response to ustekinumab induction: Results from UNIFI 2-year long-term extension
B.E. Sands1, M.T. Abreu2, R.W.L. Leong3, C. Marano4, C.D. O’Brien4, H. Zhang5, Y. Zhou5, J. Johanns5, D. Rowbotham6, T. Hisamatsu7, R.P. Arasaradnam8, E. Scherl9, S. Danese10, L. Peyrin-Biroulet11, UNIFI Investigators
1Department of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, USA, 2Department of Gastroenterology, University of Miami Miller School of Medicine, Miami, USA, 3Department of Gastroenterology, The University of New South Wales and Macquarie University, Sydney, Australia, 4Department of Immunology, Janssen Research and Development- LLC, Spring House, USA, 5Department of Clinical Biostats, Janssen Research and Development LLC, Spring House, USA, 6Department of Gastroenterology and Hepatology, Auckland City Hospital, Auckland, New Zealand, 7Department of Internal Medicine, Kyorin University, Tokyo, Japan, 8Department of Gastroenterology, University Hospital Coventry, Warwickshire, UK, 9Jill Roberts Center for Inflammatory Bowel Disease, Weill Cornell Medicine, New York, USA, 10Department of Gastroenterology, Humanitas Research Hospital, Milan, Italy, 11Department of Gastroenterology, Nancy University Hospital, Nancy, France
Background
Ustekinumab (UST) has been shown to induce and maintain clinical response and remission in moderate–severe UC patients in the UNIFI study. In the UNIFI maintenance study, the primary randomised population consisted of patients who were in clinical response 8weeks after UST induction treatment. Patients who had a delayed response to induction were also eligible for the maintenance study but were not included in the randomised analysis set. Here, we present the efficacy and safety of UST maintenance among delayed responders who were treated in the UNIFI long-term extension(LTE).
Methods
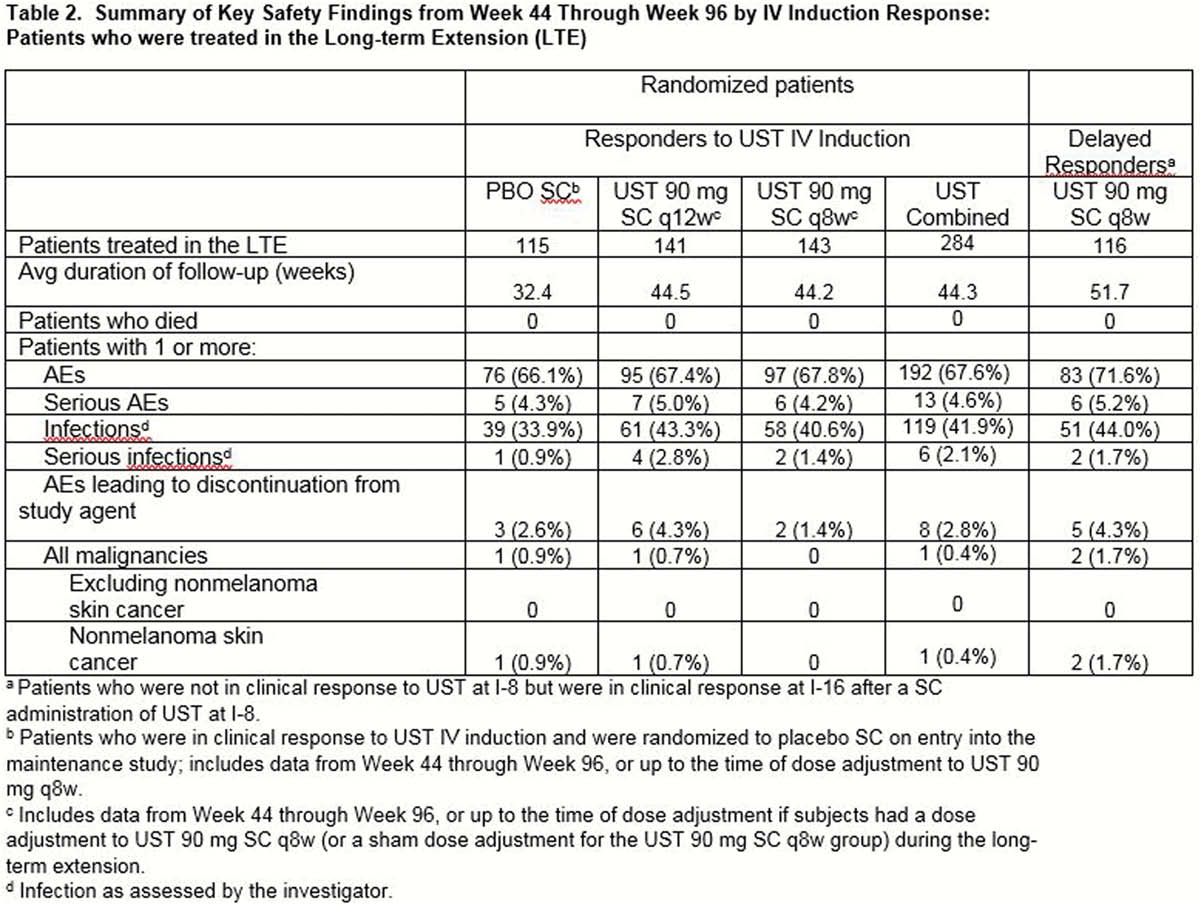
UNIFI was a single protocol of induction and randomised withdrawal maintenance studies in patients with moderate–severe UC who failed conventional or biologic therapy(including TNF antagonists and/or vedolizumab). Delayed responders were patients who were not in clinical response to UST IV induction at Week8 but achieved response at Week16 following a single UST90mg SC dose at Week8. These patients entered the maintenance study and continued to receive UST90mg SC q8w. Patients who completed Week44 evaluations were eligible to enter LTE continuing to receive UST90mg SC q8w. Efficacy in delayed responders to UST(who were treated in LTE) was evaluated over time through Week92 and safety through Week96.
Results
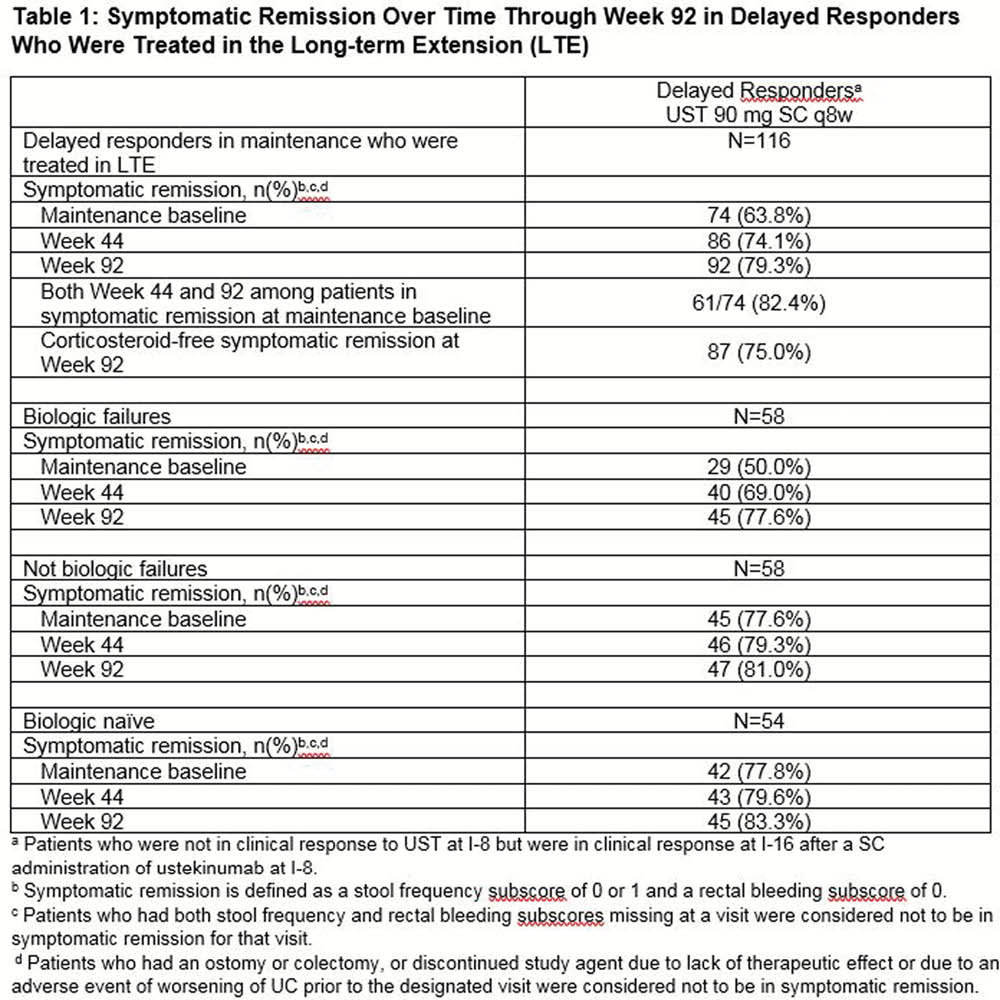
116 delayed responders to UST induction were treated in the LTE, including 58 who had previously failed biologic therapy and 58 who had not failed biologic therapy, 54 of whom were bio-naïve. Symptomatic remission(defined as stool frequency subscore of 0 or 1 and a rectal bleeding subscore of 0) rates in delayed responders to UST induction increased from maintenance baseline to Weeks 44 and 92 (Table 1). Similar trends were seen within the biologic failure subgroups; although, rates among patients who had not failed biologics (primarily bio-naïve) were greater than those of patients who had failed biologics. Among the 79.3% (
Conclusion
Delayed responders to UST treatment maintained symptomatic remission through Week 92 with UST90mg SC q8w and the majority did so in the absence of corticosteroids.


