P567 Impact of oral ferric maltol and IV iron on health-related quality of life in patients with iron deficiency anaemia and inflammatory bowel disease, and relationship with haemoglobin and serum iron
S. Howaldt1, I. Jacob2, M. Sampson3, F. Akriche4
1MZV für Immunologie, Gastroenterology, Hamburg, Germany, 2Health Economics and Outcomes Research Ltd., Health economics, Cardiff, UK, 3Shield Therapeutics PLC, Medical, London, UK, 4Norgine Ltd., Medical, Rueil Malmaison, France
Corrigendum
In the originally published version of this poster presentation, the below corrections have been made.
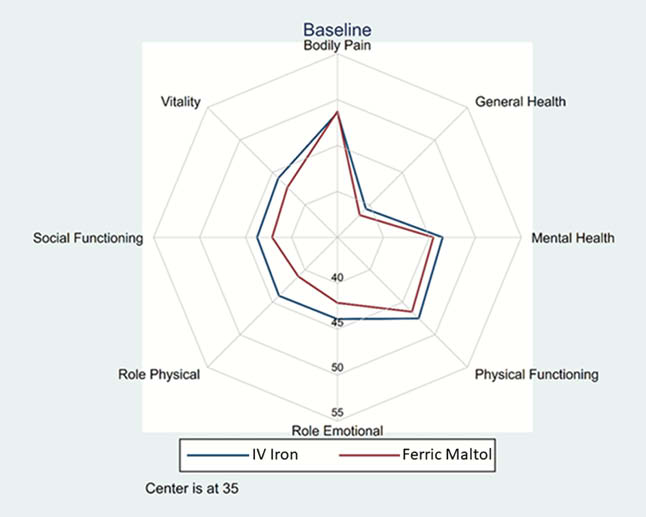
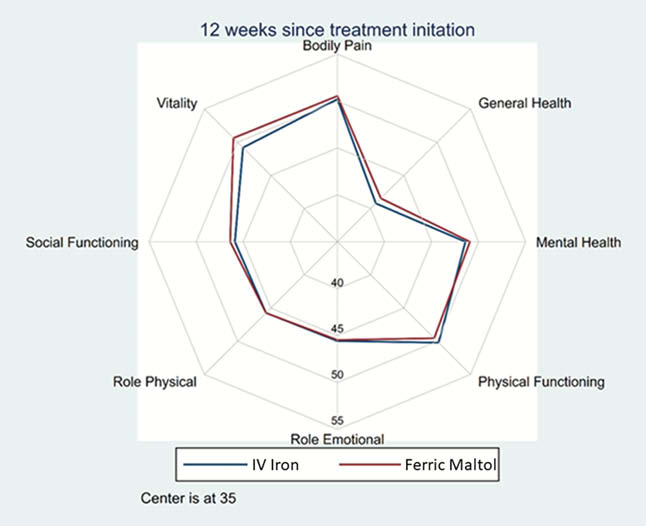
Upon the original publication, the Results section should read: “250 patients were randomised: 125 to FM and 125 to IV FCM. The primary endpoint was not met. Hb, serum iron and HRQoL all improved following both treatments at Week 12. Improvements in SF-36 physical component summary (PCS) and mental component summary (MCS) scores were slightly greater with FM (difference not statistically significant; Table 1). HRQoL improved across all SF-36 domain scores with both FM and FCM, with no statistically significant differences between treatments (Figure 1). HRQoL (MCS and PCS) improvements were positively associated with increases in Hb and serum iron (Table 1).”.
Upon the original publication, the Conclusion should read: “FM provides HRQoL benefits at least as great as those with IV FCM, and may, therefore, provide an oral alternative to IV iron in patients with IBD.” instead of “FM provides non-inferior efficacy to IV FCM with at least as great a benefit to HRQoL and may, therefore, provide an oral alternative to IV iron in patients with IBD.”.
Background
Iron deficiency anaemia (IDA) is common in inflammatory bowel disease (IBD) and can significantly impair health-related quality of life (HRQoL). IV iron is currently the main treatment for patients intolerant or unsuitable for standard oral iron. Ferric maltol (FM), a stable oral complex of ferric iron and maltol, is designed to provide efficient iron delivery and minimise formation of free iron in the gut, thus reducing the potential for gastric adverse events. The HRQoL benefits of FM and ferric carboxymaltose (FCM) and their relationship to haematological parameters were analysed using data from a randomised controlled trial.
Methods
Patients with IBD and IDA were randomised to FM (30 mg b.i.d) or IV FCM (as per local SmPC) in an open-label, Phase 3b non-inferiority study (primary endpoint haemoglobin [Hb] responder rate [proportion of patients with ≥2 g/dl increase or normalisation of Hb at week 12]; non-inferiority margin 20%). HRQoL was assessed via the Short Form Health Survey (SF-36). In a post-hoc analysis of patient-level data, Hb, serum iron and HRQoL at baseline and week 12 were summarised descriptively and correlations between HRQoL and haematological parameters were assessed via Pearson’s correlation coefficient (PCC).
Results
250 patients were randomised: 125 to FM (per-protocol [PP]
| +3.23 | 0.22 | +3.89 | 0.27 | 0.227 | 0.125 | 0.105 | 0.091 | |
| +2.09 | – | +2.52 | – | – | – | – | – |
* vs. IV FCM; †Correlations assessed via PCC, FM and IV FCM combined analysis.
Conclusion
FM provides non-inferior efficacy to IV FCM with at least as great a benefit to HRQoL and may, therefore, provide an oral alternative to IV iron in patients with IBD.


