P571 Optimal biologic management of endoscopic postoperative recurrence following ileocecal resection in Crohn’s disease
Bachour, S.(1);Shah, R.(1);Lyu, R.(2);Rieder, F.(1);Cohen, B.(1);Qazi, T.(1);Lashner, B.(1);Achkar, J.P.(1);Philpott, J.(1);Lightner, A.(3);Holubar, S.(3);Regueiro, M.(1);Click , B.(1);
(1)Cleveland Clinic Foundation, Department of Gastroenterology and Hepatology, Cleveland, United States;(2)Cleveland Clinic Foundation, Department of Quantitative Health Sciences, Cleveland, United States;(3)Cleveland Clinic Foundation, Department of Colorectal Surgery, Cleveland, United States
Background
Endoscopic postoperative recurrence (POR) of Crohn’s Disease (CD) following ileocolonic resection (ICR) is common; however, optimal treatment strategies of identified POR are unknown. We assessed the role of biologic therapy to treat endoscopic POR in a real-world cohort.
Methods
Retrospective cohort study of adult CD patients who underwent ICR from 2009-2020 at a tertiary center. Patients with endoscopic POR detected on postoperative colonoscopy and a subsequent follow-up colonoscopy were included. Patients were categorized by biologic therapy at time of POR and further sub-grouped by therapy modification after POR detection (no change, therapy optimization, or change in biologic class). Therapy optimization included: starting or modifying immunomodulator therapy, corticosteroids, or budesonide. POR was defined by Rutgeerts’ ≥ i2b.
Results
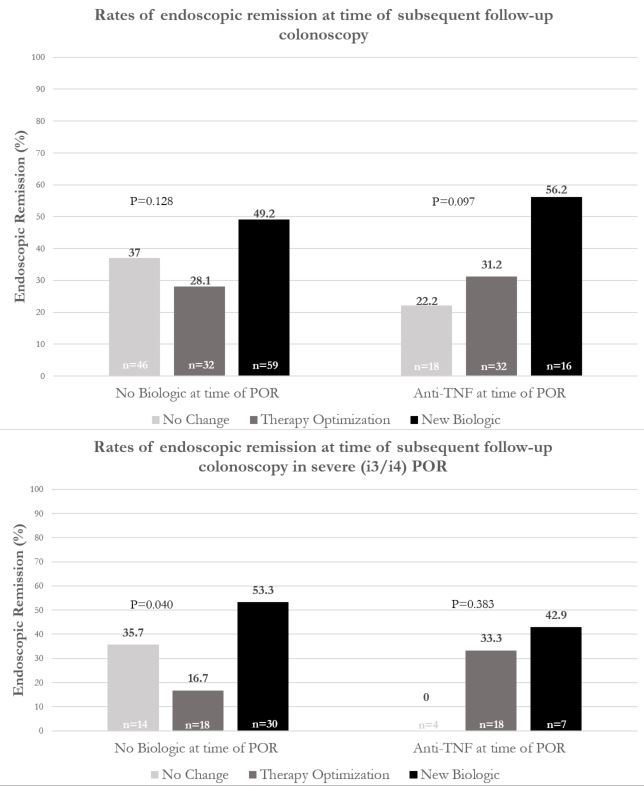
203 CD patients (49.8% female, 15.4% > 1 prior ICR, 49.0% pre-operative biologic exposure) were included. Of these, 137 (67%) patients were not on biologic therapy at POR detection: 43% subsequently started a biologic, 23% optimized therapy, and 34% had no change. 66 (33%) patients were on anti-TNF at POR identification: 24% subsequently changed biologic class, 48% optimized anti-TNF, and 27% had no change (Figure 1). There was no difference in median time from ICR to POR detection (483 days, p=0.08) or inter-colonoscopy interval (483 days, p=0.25) between groups. In patients not on biologics at POR detection, those who started a biologic saw a 21% increase in subsequent endoscopic remission compared to those who optimized therapy (49.2% vs 28.1%, p=0.09) and a 12% increase compared to those who received no change (49.2% vs 37%). In patients not on biologics with severe POR (i3/i4, n=62), there was significantly higher remission rate by starting biologic therapy compared to optimizing existing therapy (53.3% vs 16.7%) or no change (53.3% vs 35.7%), p=0.04. In individuals receiving anti-TNF at time of POR, there was a 25% increase in endoscopic remission in patients who switched biologic class compared to those who optimized therapy (56.2% vs 31.2%) and a 34% increase compared to those with no change (56.2% vs 22.2%), p=0.1. Furthermore, significantly higher rates of improved Rutgeerts’ score were observed in switching biologic class compared to therapy optimization (68.8% vs 43.8%) or no change (68.8% vs 27.8%), p=0.04.
Conclusion
After endoscopic POR detection following ICR, initiating biologic therapy in individuals not previously receiving it, and changing mechanism of action in those already receiving anti-TNF, may improve clinical outcomes compared to alternative management strategies. If confirmed, these findings may inform optimal management strategies for endoscopic POR.
Figure 3
Figure 4