P577 Mucosal eosinophil abundance in non-inflamed colonic tissue predict response to vedolizumab induction therapy in inflammatory bowel disease
Gabriëls, R.Y.(1);Bourgonje, A.R.(1);von Martels, J.(1);Blokzijl, T.(1);Weersma, R.(1);Galinsky, K.(2);Julius, J.(2);Faber, K.N.(1);Kats-Ugurlu, G.(3);Dijkstra, G.(1);
(1)University Medical Center Groningen, Department of Gastroenterology and Hepatology, Groningen, The Netherlands;(2)Takeda Pharmaceutical Company Ltd, Research, Massachusetts, United States;(3)University Medical Center Groningen, Department of Pathology and Medical Biology, Groningen, The Netherlands;
Background
Vedolizumab has shown efficacy, safety and tolerability as treatment for patients with inflammatory bowel disease (IBD). However, vedolizumab induction therapy only shows clinical response and remission in roughly 55% and 30% of IBD patients, respectively. Vedolizumab binds and blocks migration of T-lymphocytes and eosinophils. In this study, we aimed to explore the predictive value of mucosal eosinophils and serum eotaxin-1, an eosinophil chemoattractant, regarding response to vedolizumab induction therapy.
Methods
84 IBD patients treated within the University Medical Center Groningen (UMCG) (37 Crohn’s disease [CD], 47 ulcerative colitis [UC]) were included. In a subset of 24 IBD patients (9 CD, 15 UC) histopathological data were analyzed for eosinophil counts in high power fields (hpf) in non-inflamed colon ascendens tissue prior to vedolizumab treatment. In another subset of 64 IBD patients, (28 CD, 36 UC) baseline serum eotaxin-1 was quantified prior to vedolizumab treatment. Clinical response or remission was defined as a decrease of the Harvey Bradshaw Index (HBI) for CD or Simple Clinical Colitis Activity Index (SCCAI) for UC together with physician’s global assessment (PGA). Serum eotaxin-1 was externally assessed as a biomarker for response to vedolizumab induction therapy in 100 IBD patients derived from the GEMINI 1 & 2 trials.
Results
Baseline eosinophil mucosal count was significantly higher in vedolizumab induction therapy responders, compared to primary non responders (69[34-138] vs. 24[18-28] eosinophils/hpf respectively, P<0.01). Baseline serum eotaxin-1 levels in the UMCG cohort were significantly elevated in therapy responders, compared to primary non-responders (0.33 vs. 0.20 ng/mL, P<0.01). The final prediction model based on mucosal eosinophil count showed an area under the curve (AUC) of 0.90 and serum eotaxin-1 an adjusted AUC of 0.79 The optimal with balanced cut-off value for eosinophil count was > 30 eosinophils/hpf with a sensitivity of 90.9% and specificity of 92.3% (Youden’s index 0.83). Results derived from the GEMINI I & II cohorts did not show any associations between eotaxin-1 levels and therapy response.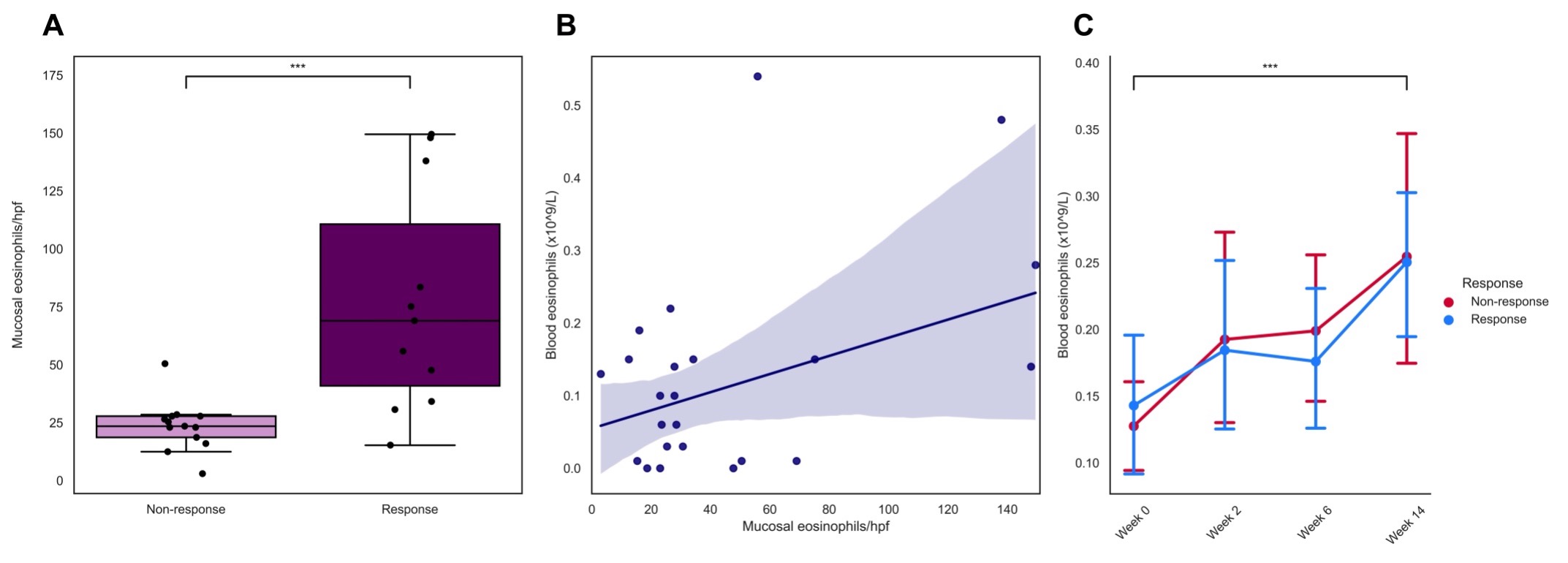

Conclusion
Mucosal eosinophil abundance in non-inflamed colon ascendens biopsies can predict vedolizumab induction therapy response in IBD patients. More studies are warranted to confirm these preliminary results and further investigate the additional value of eotaxin-1 regarding predicting vedolizumab therapy response.


