P577 Ustekinumab in actual clinical practice: our centre experience
J.M. LOPEZ TOBARUELA, A.D. Sanchez-Capilla, E.J. Ortega-Suazo, M.C. Fernandez-Cano, M. Herrador-Paredes, M.J. Cabello-Tapia, M.M. Martin-Rodriguez
Hospital Universitario Virgen de las Nieves, Gastroenterology, Granada, Spain
Background
Crohn’s disease is an entity with wide variability in its clinical manifestations, which mainly affects the gastrointestinal tract, and may also involve another organs. We have a wide variety of treatments available, being the most recent ones biological therapies. One of them is ustekinumab, which due to its innovative mechanism of action has improved the quality of life of many patients with an excellent safety profile.
Our objective was to analyze the baseline situation of 37 patients undergoing treatment with ustekinumab in our centre and the evolution of different clinical and analytical parameters at 12, 24 and 52 weeks after starting it.
Methods
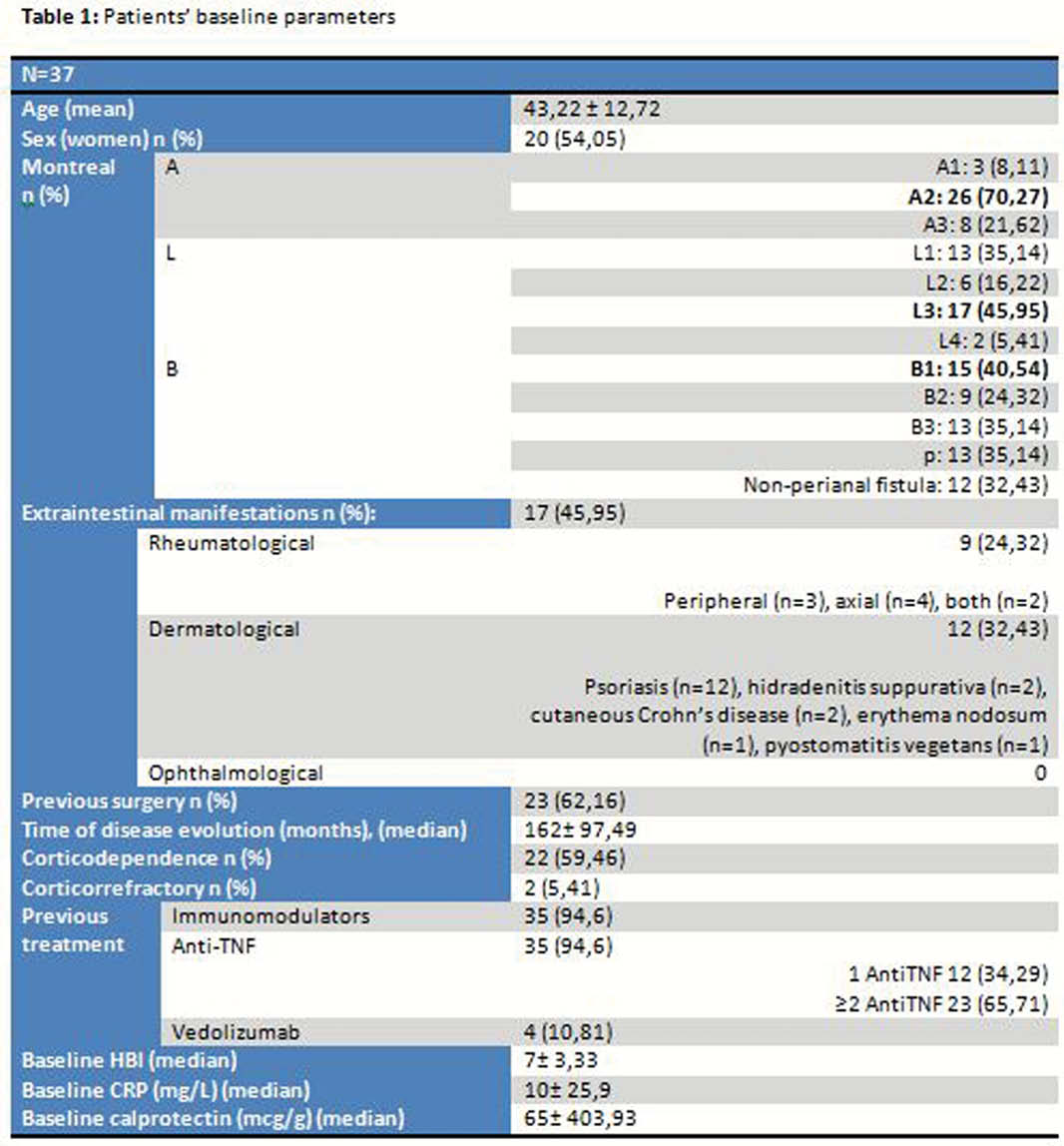
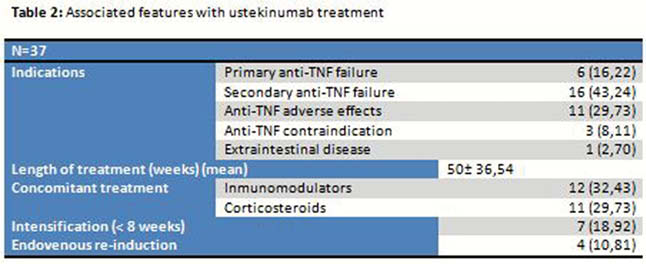
37 patients currently receiving ustekinumab treatment with indication of Crohn’s disease at Virgen de las Nieves Hospital were selected (Tables 1 and 2). Clinical response was analyzed according to the Harvey–Bradshaw Index (HBI) (clinical remission <4, clinical response decrease ≥3 points above the baseline), blood and stool test (CRP and calprotectin) at weeks 12, 24 and 52, need for surgery and safety profile (
Results
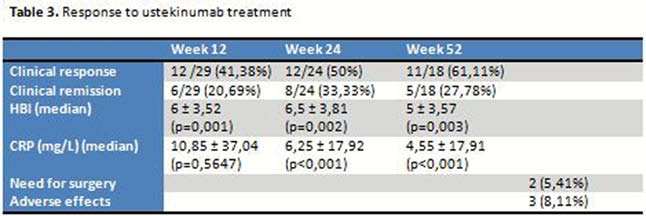
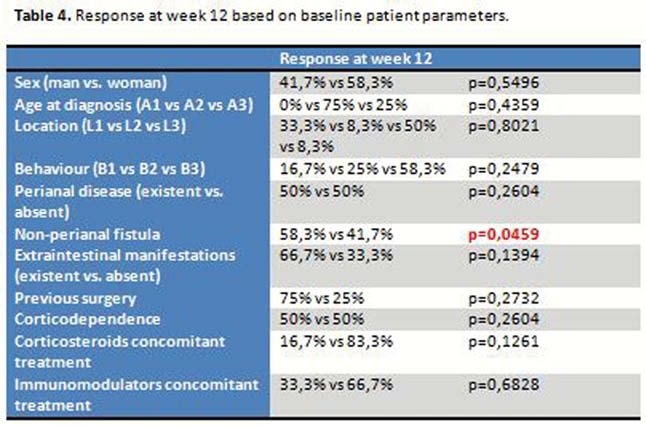
There are no significant differences in HBI or baseline CRP depending on previous or not treatment with anti-TNF or vedolizumab. No differences in HBI, CRP at weeks 12, 24 and 52 depending on if previous treatment with anti-TNF or vedolizumab. The decrease in the median values of HBI was statistically significant at weeks 12, 24 and 52; as well as CRP values at weeks 24 and 52 (
According to HBI, clinical response was obtained in 41,38%, 50% and 61,11% of patients at weeks 12, 24 and 52 and clinical remission in 20,69%, 33,33% and 27,78% respectively (
Conclusion
In this group of patients potentially difficult to treat (62% previous surgery, long-term disease, 95% previous treatment with immunomodulators and/or biological therapies (66% ≥2 anti-TNF), etc.), ustekinumab achieves clinical response in 41,38%, 50%, 61,11% at week 12, 24 and 52. Surgery was required in 2 cases and only 3 patients suffered relevant adverse effects. In a pivotal study of ustekinumab UNITI-1, 50,6% of patients who received the induction dose of 6 mg/kg had previously been treated with ≥2 anti-TNF, in our group, 65,71%. In our cohort, only 29,73% of patients received corticosteroids concomitantly at the start of ustekinumab treatment vs. 43,3% at UNITI-1. Despite these differences against our group, clinical response and remission data are similar to UNITI-1 (41,38% vs. 37,8% and 20,69% vs. 20,9% respectively). Prospective studies with more patients could identify who would benefit most from this treatment.


