P585 Duration of response to ozanimod after treatment withdrawal: results from the phase 3 True North study
Vermeire, S.(1)*;Abraham, B.(2);Bressler, B.(3);Reinisch, W.(4);Jain, A.(5);Memaj, A.(5);Akukwe, L.(5);Liu, W.J.(5);Osterman, M.(5);Canavan, J.B.(5);Sands, B.E.(6);
(1)University Hospitals Leuven, Chronic Diseases and Metabolism, Leuven, Belgium;(2)Houston Methodist Academic Institute, Gastroenterology, Houston, United States;(3)University of British Columbia, Medicine, Vancouver- BC, Canada;(4)Medical University of Vienna, Gastroenterology and Hepatology, Vienna, Austria;(5)Bristol Myers Squibb, Clinical Research, Princeton, United States;(6)Icahn School of Medicine at Mount Sinai, Gastroenterology, New York, United States;
Background
The phase 3 True North randomised trial demonstrated the efficacy and safety of ozanimod (OZA) in patients with moderately to severely active ulcerative colitis (UC). Temporary discontinuation of UC treatment can occur in clinical practice. Understanding the duration of response after treatment discontinuation can assist in clinical decision making. This analysis examined time to loss of OZA response in patients who discontinued OZA and switched to placebo (PBO) in the True North maintenance period (MP).
Methods
In True North, patients were randomised to once-daily oral OZA 0.92 mg or PBO or to open-label OZA during a 10-week induction period (IP). Patients with clinical response to OZA at Week 10 were rerandomised to OZA (OZA/OZA) or PBO (OZA/PBO) in the MP through Week 52. Disease relapse was defined as an increase in partial Mayo score (PMS) of ≥2 points vs Week 10 with absolute PMS ≥4, endoscopic subscore ≥2, and exclusion of other causes of increased disease activity unrelated to UC. A subanalysis compared outcomes in OZA/OZA and OZA/PBO patients who entered the MP after achieving clinical remission (CRM) or clinical response (CRS only, excluding patients in CRM) at Week 10.
Results
In the True North MP, 230 patients continued OZA (CRM, n=82; CRS only, n=148) and 227 patients switched to PBO (CRM, n=79; CRS only, n=148). Baseline characteristics were generally similar between groups. Patients in CRS only at Week 10 had a numerically higher proportion with severe disease and prior immunosuppressive medication use vs CRM patients (Table). Differences in disease relapse between groups became apparent after MP Week 8, after which a significantly higher proportion of OZA/OZA patients were without relapse vs OZA/PBO patients (Figure). MP Week 42 Kaplan-Meier estimates of no relapse were 86.1% and 62.6% for OZA/OZA and OZA/PBO patients, respectively. Additionally, patients in CRM at Week 10 had higher rates of nonrelapse during the MP vs those in CRS only at MP entry. Kaplan-Meier estimates of nonrelapse at MP Week 42 were 67.9% and 59.7% in OZA/PBO patients with CRM and CRS, respectively, and 90.9% and 83.4% in OZA/OZA patients with CRM and CRS, respectively. 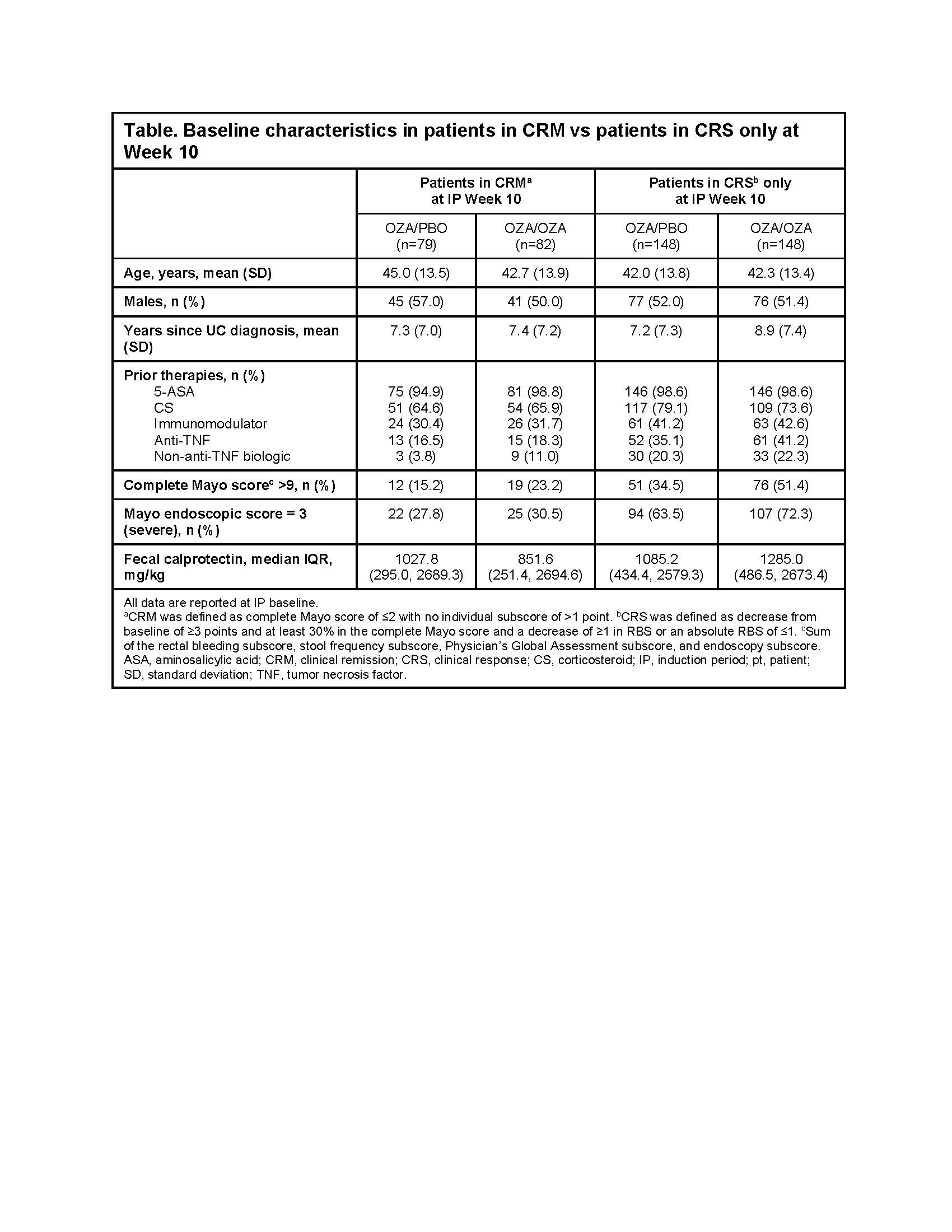

Conclusion
This analysis shows that OZA is effective at preventing disease relapse over 42 weeks of maintenance treatment in patients who achieve clinical response at Week 10. These data show that OZA maintains disease control even in the event of temporary discontinuation. However, extended discontinuation should be minimized in clinical practice.


