P588 vedolizumab trough levels at week 6 and 14 correlate with clinical remission in inflammatory bowel disease patients
M. Marino1, R. Domenis2, L. Biribin1, A. Cifù2, L. Navarria1, G. Scardino1, M. Fabris2
1Department of Gastroenterology, Azienda Sanitaria Universitaria Integrata di Udine, Udine, Italy, 2Istituto di Patologia Clinica, Azienda Sanitaria Universitaria Integrata di Udine, Udine, Italy
Background
Vedolizumab (VDZ) is a therapeutic monoclonal antibody against α 4β 7 integrin approved for the treatment of inflammatory bowel diseases (IBDs). A relationship between VDZ exposure and clinical efficacy has been highlighted by several studies, but data are still controversial. In this perspective, our study aims to investigate the clinical usefulness of VDZ drug monitoring during the induction phase to support therapeutic management of IBD patients in the real life.
Methods
This retrospective study focus on a single cohort of 25 IBD patients (7 with Crohn’s disease and 18 with ulcerative colitis) treated with VDZ between 2016 and 2019. VDZ trough levels (TLs) and anti-VDZ antibodies (AVA) were measured before infusion at weeks 6, 14 and 22, using Promonitor® ELISA assays (Grifols). Disease activity was evaluated using international criteria, defining clinical remission when Mayo Partial Score <2 and Harvey–Bradshaw Index <5.
Results
VDZ TLs showed a significant inverse correlation with disease activity at each time point (week 6: Spearman’s rho = −0.659,
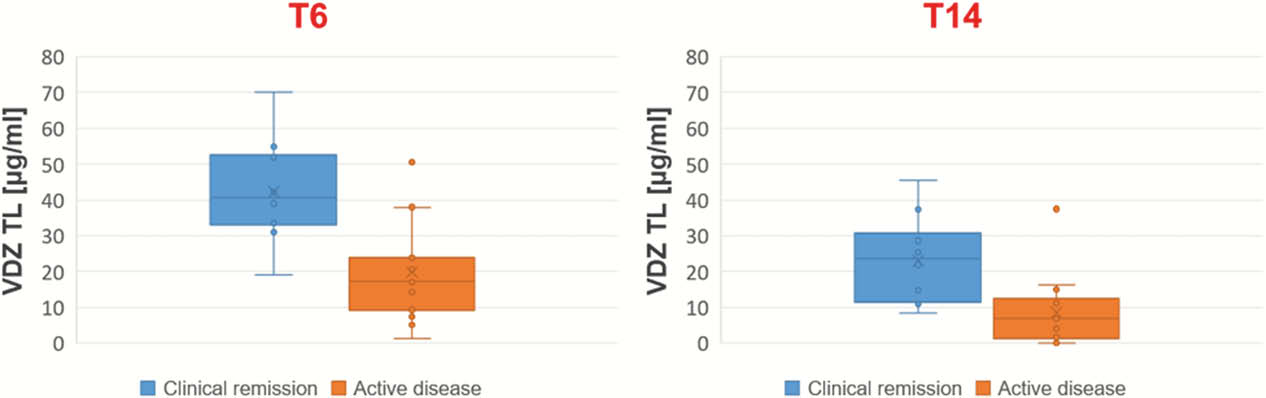
Conclusion
VDZ TLs in the induction phase correlate with the clinical score in IBD patients, helping the management of the therapeutic efficacy of VDZ, from the very beginning of the biological treatment. In particular, at week 6, VDZ trough levels > 24 µg/ml may represent a potential drug concentration target to achieve long-term remission.


