P589 Longer-term outcomes in patients with Crohn’s disease who are primary non-responders to anti-TNF in a single UK centre
P. HODGES, J. Saunders, F. Betteridge
Department of Gastroenterology, Royal United Hospitals Bath NHS Foundation Trust, Bath, UK
Background
Primary non-response (PNR) is reported to occur in up to 24% patients with inflammatory bowel disease treated with anti-TNF drugs. The longer-term outcomes of these patients are less well documented however it is recognised that this group represents a significant treatment challenge. We report retrospective outcomes for these patients from a single centre.
Methods
All patients with Crohn’s disease who stopped anti-TNF therapy because of PNR from 2013 to 2017 were identified from our database. Data were retrospectively collected from electronic patient records.
Results
124 patients were started on anti-TNF therapy in this 4-year period and PNR occurred in 26 of these (21%). Median follow-up was 43.5 months.
| No. patients with PNR | 26 |
| No. patients on infliximab | 14 |
| No. patients on adalimumab | 12 |
| Median age | 43 |
| Median duration of disease (years) | 3.5 |
| Median duration of treatment (months) | 6 |
| Distribution of disease | |
| Small bowel | 4 |
| Colonic | 12 |
| Ileocolonic | 9 |
| Unknown extent | 1 |
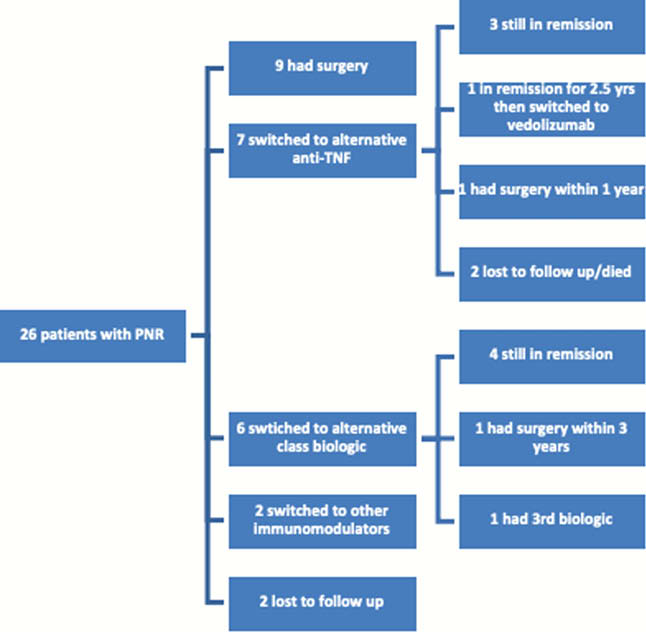
Following PNR 9 (34.6%) patients had surgery as next treatment although in the majority of these cases (6/9) this was before alternative class biologic drugs were available. 7 (26.9%) switched to an alternative anti-TNF again with the majority of these (5/7) being prior to the availability of other biologic class drugs. The subsequent outcomes for these patients are; 3 remain in remission, 1 failed to respond to an alternative anti-TNF and went on to have surgery within 1 year, 1 was in remission for 2 years then switched to vedolizumab, 2 were lost to follow-up or died for unrelated reasons. 6 (23.1%) switched to a different biologic class. Of these, 4 remain in remission, 1 required subtotal colectomy within 3 years, and 1 had 3 further biologic agents all of which failed and is now awaiting a trial drug or surgery. 2 (7.7%) switched to other immunomodulators. Two (7.7%) were lost to follow-up.
Conclusion
Overall 11 of the 26 (42%) patients required surgery within 3 years of primary anti-TNF failure. These data span a period during which vedolizumab and ustekinumab became available; therefore, the proportion of patients requiring surgery as next intervention is likely to be less now that these alternative drugs are available. In this cohort 4/6 patients who received alternative biologic class drugs who would previously have gone on to have surgery as the next intervention remains in remission (median follow-up 37 months). Although this is a small cohort, PNR response is shown to be a marker of poor prognosis with the availability of alternative biologics appearing to reduce the short-and medium-term need for surgery.


