P594 Vedolizumab dose intensification does not always improve treatment persistence.
Hilley, P.(1);Peterson, A.R.(1);Wong , D.(1,2);Hine, K.(1);Srinivasan, A.(1);Choy, M.C.(1,2);De Cruz, P.(1,2);
(1)Austin Health, Gastroenterology, Melbourne, Australia;(2)University of Melbourne, Department of Medicine- Austin Academic Centre, Melbourne, Australia;
Background
Vedolizumab is effective in inducing and maintaining remission in patients with both Crohn’s Disease (CD) and ulcerative colitis (UC). Recent data has demonstrated that there is superior persistence with vedolizumab compared to anti-TNF biologics. However, the efficacy of dose intensified vedolizumab remains uncertain. We aimed to assess whether vedolizumab dose intensification resulted in a meaningful improvement in treatment persistence and to determine whether there were any clinical, biochemical or endoscopic parameters that were associated with a response to dose intensification.
Methods
A retrospective analysis of all patients with CD or UC that had received dose intensified vedolizumab every 4 weeks, was undertaken at an Australian tertiary, metropolitan IBD service between the 1st of July 2015 and 15th of November 2021. Electronic medical records were reviewed to retrospectively calculate the partial Mayo score (pMS) for UC and Harvey-Bradshaw Index (HBI) for CD, as well as document the C-Reactive Protein (CRP), Faecal Calprotectin (FC) and endoscopic disease assessment. Data were obtained at commencement of vedolizumab, time of dose escalation, as well as at 6 and 12 months post escalation. The primary outcome was treatment persistence at 6 months post escalation.
Results
A total of 33 patients required dose intensification to address primary non-response or secondary loss of response. Median time to escalation was 9 months (IQR 3-14). 11/33 (33%) remained on escalated dosing at the end of the study period. 4/11 (36%) were escalated prior to, and 7/11(32%) after week 14. Escalated therapy was continued for a median of 5 months (IQR 3-12).
Treatment persistence was 45% at 6 months, and 24% at 12 months. In patients who had vedolizumab ceased at 6 months post escalation, there was a rate of clinical activity of 93%, biochemical activity of 93% and endoscopic activity of 100%. For those maintained on vedolizumab at 6 months post intensification the rates were 50% for both clinical and biochemical activity. No clinical/biochemical/endoscopic parameters were associated with response and no statistically significant difference was noted in disease activity following escalation.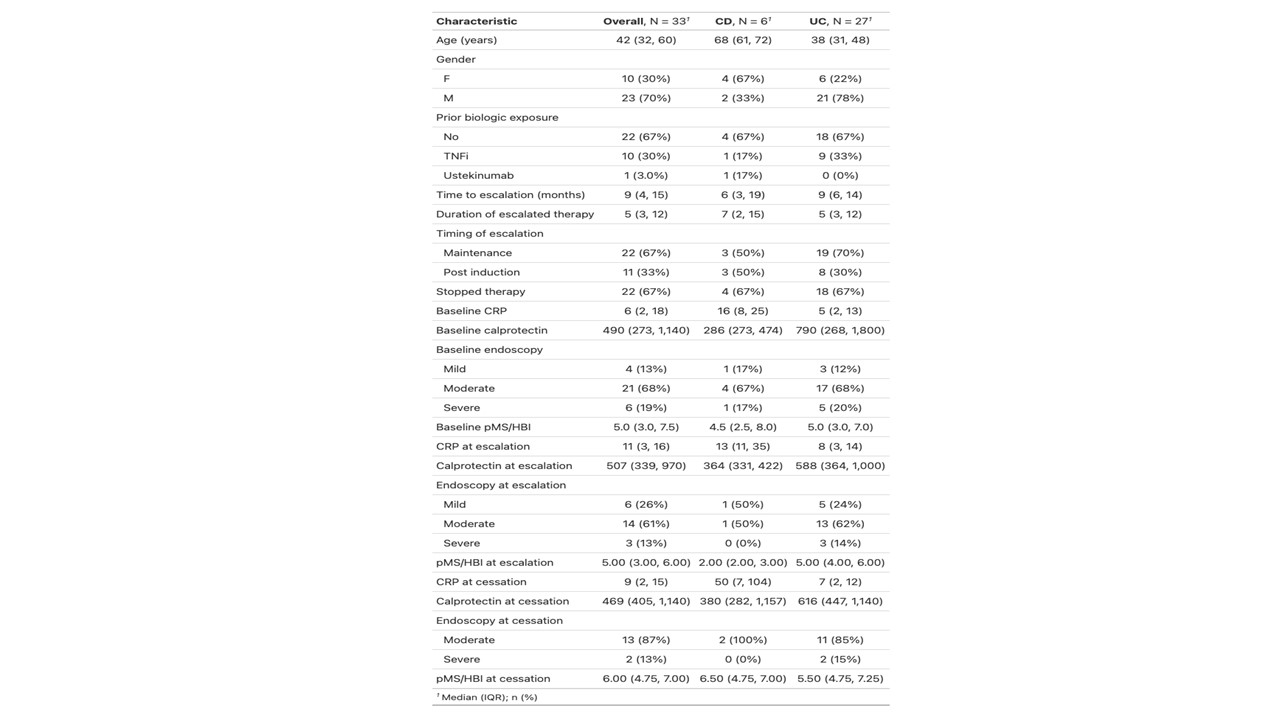

Conclusion
There was a low rate of treatment persistence for patients undergoing vedolizumab dose intensification. Rates of disease activity at the time of cessation for patients who discontinued vedolizumab were high. Dose intensification of vedolizumab may not capture response nor translate into treatment persistence. Further prospective studies examining vedolizumab levels and dose intensification are warranted but these data suggest a low threshold for switching to alternative agents if response cannot be captured within 6 months of dose escalation.


