P597 Cycling Anti-TNF Therapy in Inflammatory Bowel Disease: Effectiveness and Durability of Switching from adalimumab to infliximab
Steinberg, J.(1);Andersen, M.(1);Krugliak Cleveland, N.(1);Traboulsi, C.(1);Rodriguez, T.(1);Cohen, R.(1);Dalal, S.(1);Micic, D.(1);Pekow, J.(1);Atsushi, S.(1);Rubin, D.(1);
(1)University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, United States
Background
Anti-TNF therapy remains the cornerstone of moderate to severe inflammatory bowel disease (IBD) treatment. There are few data regarding switching from subcutaneous (SubQ) to intravenous (IV) agents. We evaluated the clinical utility of cycling adalimumab (ADA) for infliximab (IFX) in IBD patients (pts) treated at a tertiary center.
Methods
This is a retrospective observational study where we evaluated pts with IBD who cycled from ADA to IFX from 2010 to 2018. Pts were followed for 1 year following IFX initiation. We reviewed baseline demographic and clinical data. Primary outcomes included changes in CRP and clinical disease activity indices which were statistically analyzed using Wilcoxon Signed Rank Test.
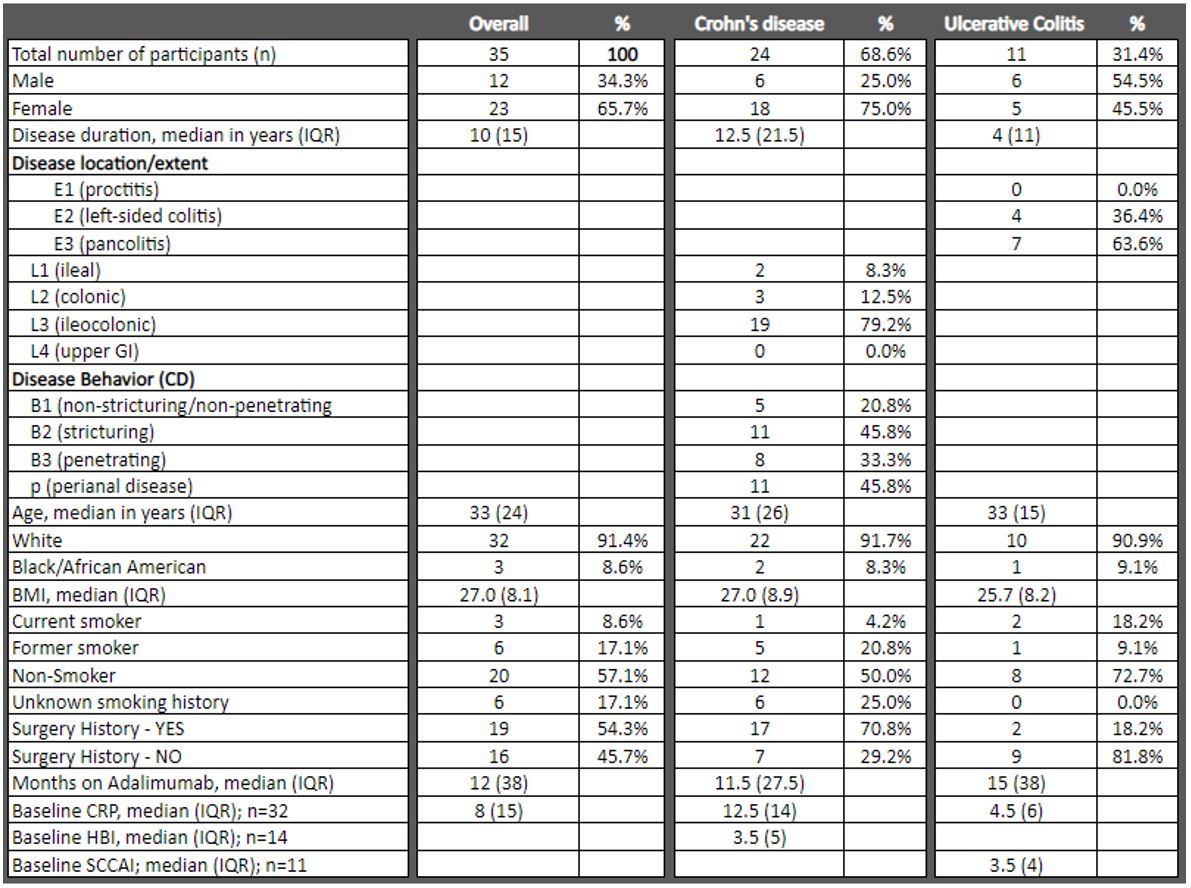
Results
24 pts with Crohn’s disease (CD) and 11 with ulcerative colitis (UC) were included. Median age at time of IFX initiation in the overall cohort was 33 years with median disease duration of 10 years. Most pts in the UC cohort had pancolitis (63.6%), while the CD cohort mostly had ileocolonic disease (79.2%). Prior to IFX initiation, median baseline CRP was 8 (IQR 15), with median HBI of 3.5 (IQR 5), and SCCAI of 3.5 (IQR 4) in the CD and UC cohorts, respectively. Additional baseline demographics are in Table 1. Following median 12 months on ADA therapy in the overall cohort, 16 pts (45.7%) discontinued ADA due to primary or secondary loss of response, 8 pts (22.8%) due to anti-drug antibody formation, and 11 pts (31.4%) due to adverse events or unclear causes. At the time of starting IFX therapy, 17 pts (48.6%) were using steroids, while 24 pts (68.6%) were on immunomodulatory therapy. In the overall cohort, after cycling to IFX, median CRP was lower at 8 weeks (4; IQR 9), 26 weeks (3; IQR 11), and 52 weeks (5; IQR 10) as compared to baseline; only the 8 week CRP reached statistical significance (P=0.0447, 0.0662, and 0.0876, respectively). In the CD cohort, median CRP was significantly lower at 8 weeks (7; IQR 6; P=0.0314) and 52 weeks (5; IQR 7; P=.0410) as compared to baseline (12.5; IQR 14). Although signals of median HBI and SCCAI scores trended lower after 8 weeks, 26 weeks, and 52 weeks of IFX therapy, these did not reach statistical significance. A total of 15 pts (43.0%) stopped IFX prior to one year. Subgroup analysis of 5 pts who developed anti-ADA antibodies demonstrated statistically significant reduction of median CRP (10; IQR 22.5; P=.043) after 52 weeks of IFX therapy. Additional results are detailed in Table 2.
Conclusion
Cycling anti-TNF therapy from ADA to IFX offers clinical utility in preserving mechanistic benefit of TNF-α blockade in IBD pts leading to improvement of CRP, particularly in CD pts and those who developed immunogenicity to ADA.