P597 Pooled safety analyses from Phase 3 studies of filgotinib in patients with rheumatoid arthritis
R. Besuyen1, D. Walker2, K. Winthrop3, M. Genovese4, B. Combe5, Y. Tanaka6, A. Kivitz7, F. Matzkies8, B. Bartok9, L. Ye10, Y. Guo10, C. Tasset1, J. Sundy11, E. Keystone12, R. Westhovens13, W. Rigby14, G. Burmester15
1Galapagos, Clinical Development, Mechelen, Belgium, 2Northumbria Healthcare, Department of Rheumatology, Northumbria, UK, 3Oregon Health and Science University, Department of Rheumatology, Portland, USA, 4Stanford University USA, Immunology and Rheumatology, Palo Alto, USA, 5CHU Montpellier University, Department of Rheumatology, Montpellier, France, 6University of Occupational and Environmental Health Japan, Department of Rheumatology, Kitakyushu, Japan, 7Altoona Center for Clinical Research USA, Clinical Research, Duncansville, USA, 8Gilead Sciences Inc., Medical Affairs, Foster City, CA, USA, 9Gilead Sciences Inc., Clinical development, Foster City, CA, USA, 10Gilead Sciences Inc., Biostatistics, Foster City, CA, USA, 11Gilead Sciences Inc., Clinical research, Foster City, CA, USA, 12Mount Sinai Hospital and University of Toronto Canada, Department of Rheumatology, Toronto, Canada, 13University UZ Leuven, Department of Rheumatology, Leuven, Belgium, 14Dartmouth College, Department of Rheumatology, Lebanon, NH, USA, 15Charité-University Medicine Berlin, Rheumatology and Clinical Immunology, Berlin, Germany
Background
Filgotinib (FIL) is an oral, selective Janus Kinase 1 inhibitor under development for the treatment of rheumatoid arthritis (RA) and other inflammatory diseases. Safety and efficacy of FIL was investigated in the FINCH clinical program, which includes three Phase 3, randomised, multicentre studies in patients with moderate to severely active RA. FINCH1: patients with inadequate response to MTX (NCT02889796); FINCH2: patients receiving conventional disease-modifying antirheumatic drugs (csDMARDs) with inadequate response to biological DMARDs (NCT02873936); FINCH3: MTX-naïve patients initiating MTX ± FIL, or receiving FIL monotherapy (NCT02886728). We present pooled safety data up to 24 weeks (W24).
Methods
The FINCH studies enrolled patients with RA (2010 ACR/EULAR criteria), ≥6 swollen joints and ≥6 tender joints at screening and Day 1. Safety analyses included patients receiving ≥1 dose of study drug. Patients in FINCH 1 and 2 who did not experience at least a 20% improvement in both swollen joint count and tender joint count by W14 discontinued study drug and switched to a standard of care. W24 safety data from all studies were aggregated and summarised. Key safety endpoints were treatment-emergent adverse events (TEAEs), serious TEAEs, TEAEs of interest, deaths and treatment-emergent laboratory abnormalities.
Results
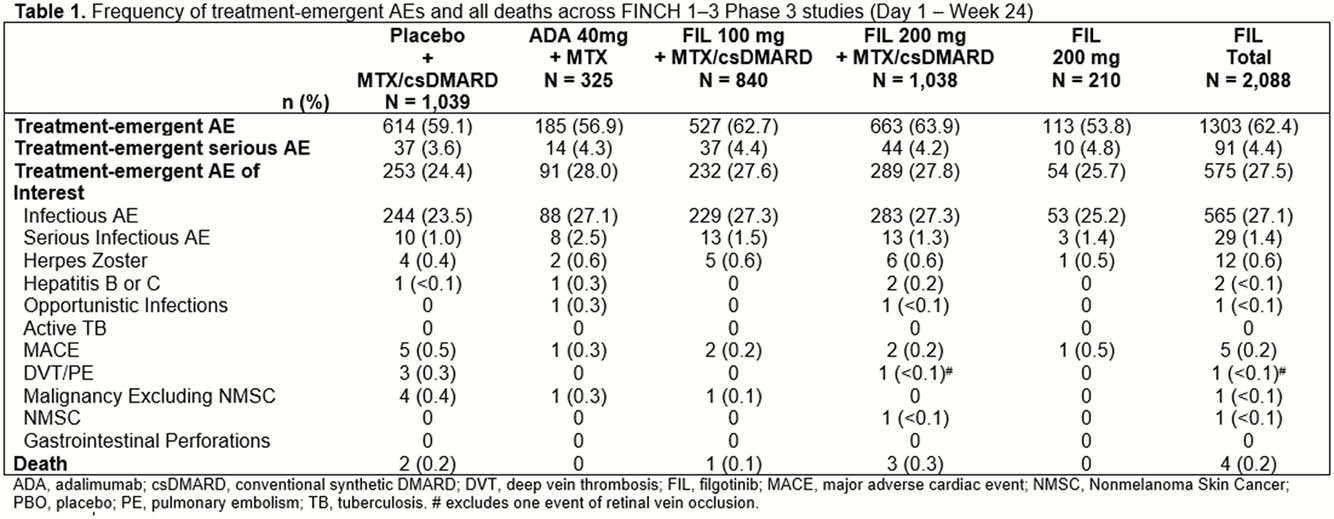
3452 patients were evaluated; 2088 received FIL. At W24, the frequency of TEAEs and TEAEs of interest were similar for those who received FIL and those in the control groups (Table 1). Most TEAEs were infections. Laboratory abnormality rates were similar between FIL and control groups, and were mild to moderate (grades 1 and 2). Overall, the frequency of major adverse cardiac events, herpes zoster virus, deep vein thrombosis and pulmonary embolism was low, and similar across groups.
Conclusion
Pooled data from this large database highlights the favourable safety and tolerability profile of FIL in patients with RA both as monotherapy and in combination with MTX/csDMARD.


