P600 Serologic response to COVID-19 vaccines in IBD patients: a prospective study
Martin ArranzPhD- MD, M.D.(1);García Ramírez, L.(2);Montero Vega, D.(3);Martín-Arraz, E.(2);Sánchez Azofra, M.(2);Poza Cordon, J.(2);Rueda Garcia, J.L.(2);Noci Belda, J.(2);Verges Martínez-Meco, T.(2);Blanco San Miguel, P.(2);Suarez Ferrer, C.(2);
(1)La Paz University Hospital. Universidad Autónoma de Madrid. IdiPaz, Gastroenterolgy, Madrid, Spain;(2)La Paz University Hospital.IdiPaz, Gastroenterolgy, Madrid, Spain;(3)La Paz University Hospital., Microbiology, Madrid, Spain;
Background
Our objective is to evaluate the serologic response to the SARS-CoV-2 vaccine in patients with Inflammatory Bowel Disease (IBD). In addition to that, we want to analyze the influence of immunosuppressive drugs in that response, as well as describe the adverse events in this population.
Methods
We included 266 patients in a unicentric prospective study. All patients signed informed consent. A serologic blood test was made days before the first dose and 2-4 weeks after the complete immunization. We used Siemens Atellica Anti-SARS-CoV-2 (N) and Vircell Virclia ( S and N) electrochemiluminescence immunoassay to detect antibodies to SARS-CoV-2). If they were discordant, the results were considered as undetermined. The IBD treatment was stable along the study. The statistics analysis of data was done with Stata 16.
Results
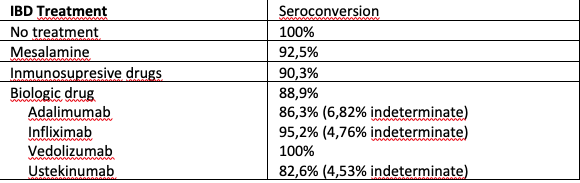
Basal characteristics are described in table 1. The patients were on treatment with: 15 (5.66%) had no treatment, 47 (17.7%) had mesalamine, 4 (1.51%) had corticosteroids, 41 (15.47%) had immunomodulator, 113 (42.64%) had biologic and 45 (16.98%) had combo. Amongst the biologic drugs: Infliximab 51 (32,3%), Adalimumab 50 (31,6%) , Vedolizumab 19 (12,03%) y Ustekinumab 31 (19,6%). The vaccines were messenger RNA BNT162b2 (Pfizer-BioNTech) in 154 patients (58%), AZD1222 (Oxford-Astra Zeneca), in 80 patients (30%), messenger RNA-1273 (National Institutes of Health [NIH]-Moderna) vaccines in 19 patients (7%) and Ad26.CoV2.S ( Janssen) in 13 patients (5%). 72.7% after first dose and 86% after second experienced side effects, with no severe cases (see table 2). Depending on the administered vaccine we can see 80% with Pfizer, 93.3% using Moderna, 49% with Astrazeneca and 100% using Janssen. This differences reached statistical significance (p=0.02). In the first blood test 222 (85%) patients tested negative, 27 patients positive (10.34%) and 12 patients undetermined (4.6%). Patients with a previous positive test were excluded. After the vaccination, 92.5% of our patients (233) had positive antibodies against SARS-COV2, a 4,75% (12 patients) undetermined titles, and a 2.75% (7 patients) were negative. In table 3we show the seroprevalence with different treatments. When analysing the antibody formation depending on the vaccine administered we can see: Pfizer 92.9%, Moderna 93.3%, Astrazeneca 98.4%, Janssen 12.5% (75% indetermined) (p< 0,0001)


Conclusion
The antibody response following Sars-CoV-2 vaccination is high in IBD patients. However, patients treated with inmunosupresive or biologic drugs (except vedolizumab) have a lower response and is significantly lower with the Ad26.CoV2.S ( Janssen) vaccine, which could influence future doses. Adverse events are frequent but not severe.


