P604 Can we reduce intravenous monoclonal antibody observation times without compromising patient safety? A single centre retrospective observational study
F. REES1, A. Packham1, C. Blake1, E. Hills1, G. Scutt2, A. St Clair-Jones1
1Brighton and Sussex University Hospitals, IBD Team- Gastroenterology, Brighton, UK, 2University of Brighton, Pharmacy, Brighton, UK
Background
Monoclonal antibodies (MAbs) are integral to inflammatory bowel disease (IBD) management. The administration of intravenous (IV) MAbs, infliximab and vedolizumab, for Brighton and Sussex University Hospital patients is via an outpatient clinic. Licensing specifies lengthy observation times; infliximab for induction (infusion 1 to 3) and maintenance (infusion 4 onwards) requires 1 to 2 h observation. Vedolizumab for induction (infusion 1 to 2) requires 2 h observation and maintenance (infusion 3 onwards) 1-h observation. This can affect waiting times; 33% UK patients waited longer than two weeks for infliximab. A reduction in observation times could improve capacity but needs to be done without compromising patient safety.
Methods
A single centre observational study was conducted. Retrospective data were collected on all current patients receiving infliximab or vedolizumab at BSUH. Data were collected over 12 weeks (April to July 2019); patients seen twice in this period were included once. The presence of reaction from current and previous infusions was determined by patient questioning and patient records review. Reaction occurrence, nature and management were recorded.
There is not a grading system for IBD infusion-related reactions. To standardise we used the cancer Common Terminology Criteria for Adverse Events; grade 3 and above is designated severe (grade 5 being death).
Results
For infliximab 130 patients were reviewed with 2607 infusions administered in total. For vedolizumab 69 patients were reviewed with 557 infusions administered in total.
| Number of Patients | Number of Infusions | Number of Infusion-related Reactions | Reaction | Infusion Number Reaction Occurred On | Grade | Symptoms | Management | Therapy Continued | ||
| Infliximab | Induction | 130 | 2607 | 3 | 1 | 1 | 2 | Itching | Chlorphenamine IV | Yes |
| 2 | 2 | 2 | Flushing and chest tightness | Hydrocortisone IV, chlorphenamine IV + paracetamol oral (PO) | No - switched | |||||
| 3 | 3 | 2 | Shortness of breath | Chlorphenamine IV | Yes | |||||
| Maintenance | 121 | 2583 | 0 | NA | NA | NA | NA | NA | NA | |
| Vedolizumab | Induction | 69 | 557 | 1 | 1 | 1 | 1 | Headache | Paracetamol PO | Yes |
| Maintenance | 64 | 550 | 0 | NA | NA | NA | NA | NA | NA |
NA, non applicable.
Due to the small sample size significance could not be reached.
The survival plot indicates high levels of ‘no reactions’ observed in the first 4 infusions of infliximab 97.7% (+1.6%, -4.7%), and first 3 infusions of vedolizumab 96.9% (+2.3%, −8.8%).
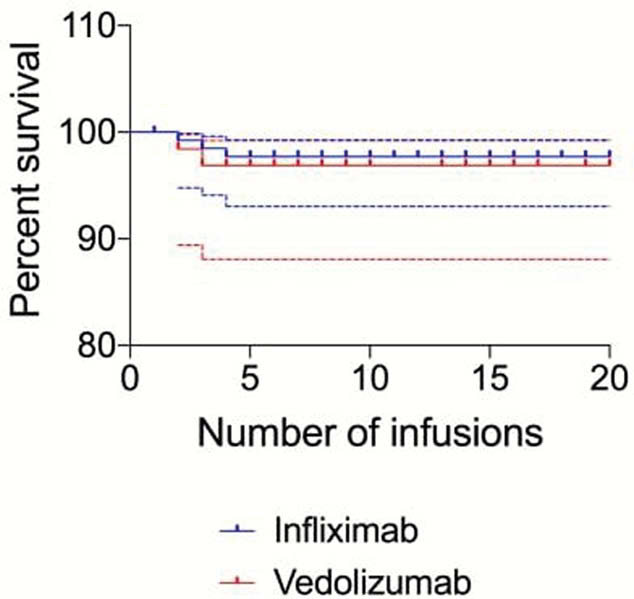
Considering capacity over 12 weeks, for infliximab a minimum of 121 could be recouped and 64 h for vedolizumab. Extrapolated this could equate to 740 h per year.
Conclusion
All reactions occurred within three infusions, were non-severe and managed within the infusion clinic. By removing the observation period from infusion 4 onwards, infusion clinic capacity could be increased but further data from multiple centres are required to prove significance.


