P610 Real-world effectiveness and safety of advanced therapies for the treatment of moderate-to-severe ulcerative colitis (UC): evidence from a systematic literature review (SLR)
Irving, P.M.(1);Hur, P.(2)*;Gautam, R.(3);Guo, X.(4);Vermeire, S.(5);
(1)Guy's and St Thomas' Hospital- Guy's and St Thomas' NHS Foundation Trust, Gastroenterology, London, United Kingdom;(2)Pfizer Inc., Inflammation and Immunology, New York, United States;(3)EVERSANA Pvt. Ltd, HEOR/Real-world Evidence, Mumbai, India;(4)Pfizer Inc., Gastroenterology- Inflammation and Immunology, Collegeville- PA, United States;(5)University Hospitals Leuven, Department of Gastroenterology & Hepatology, Leuven, Belgium;
Background
Since advanced therapies (ATs) for moderate to severe UC are increasing, their effectiveness and safety in real-world (RW) settings should be assessed.
Methods
A SLR was done as per method described by Cochrane Collaboration and PRISMA guidelines, using Embase, MEDLINE and MEDLINE-In Process databases to find RW studies of biologics (adalimumab/ADA, golimumab/GOL, infliximab/IFX, vedolizumab/VEDO and ustekinumab/UST) or small molecules (filgotinib/FIL, ozanimod/OZA and tofacitinib/TOFA) in patients (pts) with UC. Only products approved at the time of search were included. Studies published in English as full papers (Jan-2005–Feb-2022) or congress abstracts (Jan-2019–Feb-2022) were included. Studies with <30 pts or with only bio-naïve pts were excluded.
Results
Of 3930 results, 139 studies were included (18 comparative and 121 single AT). 13% of studies had only bio-exposed pts, 63% had >50% bio-exposed pts and 28% had subanalysis for bio-exposed pts.
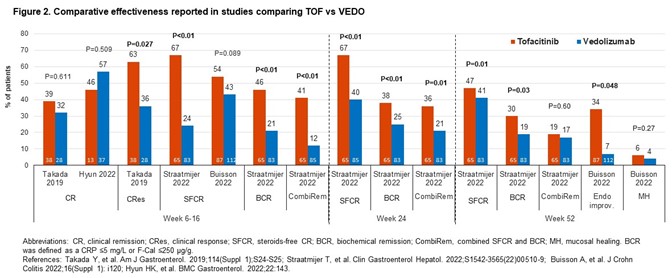
Comparative studies of VEDO vs TNF inhibitors (TNFIs; n=10); TOFA vs VEDO (4), TNFIs (3) or UST (1). In comparative effectiveness studies (16), rates of clinical remission (CR) with VEDO were numerically higher vs TNFIs (Fig1). For TOFA vs VEDO, the effectiveness of TOFA in multiple outcomes was numerically (occasionally significantly) greater (Fig2). The study of UST vs TOFA showed similar rates of steroid-free CR (Dalal 2021). In 2 studies, IFX was the most effective TNFI.
Comparative safety studies (14) mostly reported % pts with any/serious adverse events (AEs/SAEs) with limited reporting of incidence of MACE and malignancy. A study showed no significant differences in the risk of SAEs (HR0.899;95%CI0.502–1.612) or serious infections (HR1.235;95%CI0.608–2.511) for VEDO vs TNFIs (Lukin 2022). Two studies showed comparable AE rates for TOFA and VEDO. One study showed that VEDO pts had higher chance of experiencing AEs than TOFA pts (OR1.83;95%CI1.10–3.03) but similar number of SAEs (OR0.39;95%CI0.03–4.33; Straatmijer 2022). A study of TOFA vs UST showed similar overall AE rates (Dalal 2021). AE rates for TNFIs were comparable.
In single AT studies (VEDO n=50, TOFA 24, ADA 18, GOL 14, IFX 10, and UST 5), % of pts with CR and AEs/SAEs varied widely.
No studies were found for FIL and OZA.
Conclusion
In comparative studies of largely bio-exposed pts, CR rates with TOFA were generally numerically higher vs VEDO and comparable with UST; and numerically higher for VEDO vs TNFIs. IFX was most effective TNFI. SAE rates were similar across ATs. The results should be interpreted cautiously due to varied baseline characteristics though few studies were propensity scores matched. More comparative studies are needed as CR in single AT studies varied widely.


