P616 Fatigue in pediatric inflammatory bowel diseases – systematic review and rates in a prospective cohort
Focht, G.(1)*;Turner, S.(2);Carmon, N.(1);Lev-Tzion, R.(1);Orlanski-Meyer, E.(1);Ledder, O.(1);Yogev, D.(1);Assa, A.(3);Turner, D.(1);
(1)Shaare Zedek Medical Center, The Juliet Keidan Institute of Pediatric Gastroenterology and Nutrition, Jerusalem, Israel;(2)Ben Gurion University of the Negev, Ben Gurion University of the Negev, Beer Sheva, Israel;(3)Shaare Zedek Medcial Center, The Juliet Keidan Institute of Pediatric Gastroenterology and Nutrition, Jerusalem, Israel;
Background
Studies in adults suggest that fatigue is amongst the most commonly reported symptoms, reaching ~50% of patients, often even while the patients enter clinical remission. However, fatigue has been rarely discussed in children and no study hitherto determined the rate of fatigue in pediatric IBD (PIBD). We aimed to perform a systematic review of the literature in children with either CD or UC and to explore the rate of fatigue in a prospective inception cohort of PIBD.
Methods
A systematic review of the literature was conducted for pediatric studies of any design reporting fatigue as an outcome. Manuscripts were reviewed by 2 authors, inclusion was based on consensus. Next, children with new onset IBD were asked to complete the IMPACT-III, a health-related quality of life (QOL) questionnaire at 4 months after diagnosis, to capture response to treatment. Children <9 years who are unable to reliably report fatigue by themselves were excluded. Four fatigue-related questions were analyzed (Table 1). Each question has 5 response options each assigning 25 points according to the users’ guide (i.e. 0, 25, 50, 75, 100 points when the latter implies higher QOL and lack of fatigue). Remission was defined as wPCDAI<12.5 for CD and PUCAI<10 for UC. Explicit clinical and demographic data were prospectively recorded.
Results
In the systematic review only 12 studies reported on fatigue in pediatric IBD were identified,8 of which reported fatigue as primary outcome and 1 was an intervention trial. None provided rate of fatigue,also since unlike in adults,no PIBD-specific fatigue measuring scale was available. A total of 86 children were included in the inception cohort (median age 14.5[IQR 12.8-15.9] years,39[45%] females;59[69%] CD,27[31%] UC;47(55%) in clinical remission). At 4 months, only 4 patients (4.6%) selected the highest score (100) for all 4 questions, i.e. no fatigue). Forty four children (53%) had min 2 questions with a score <100,of whom 27 (61%) were in clinical remission. Twenty-eight children (33%) did not score 100 in neither of the 4 questions and 12(14%) had scored all 4 questions≤50. Of the 47 children who were in clinical remission,33(70%) had at least 2 of the 4 questions scored<100.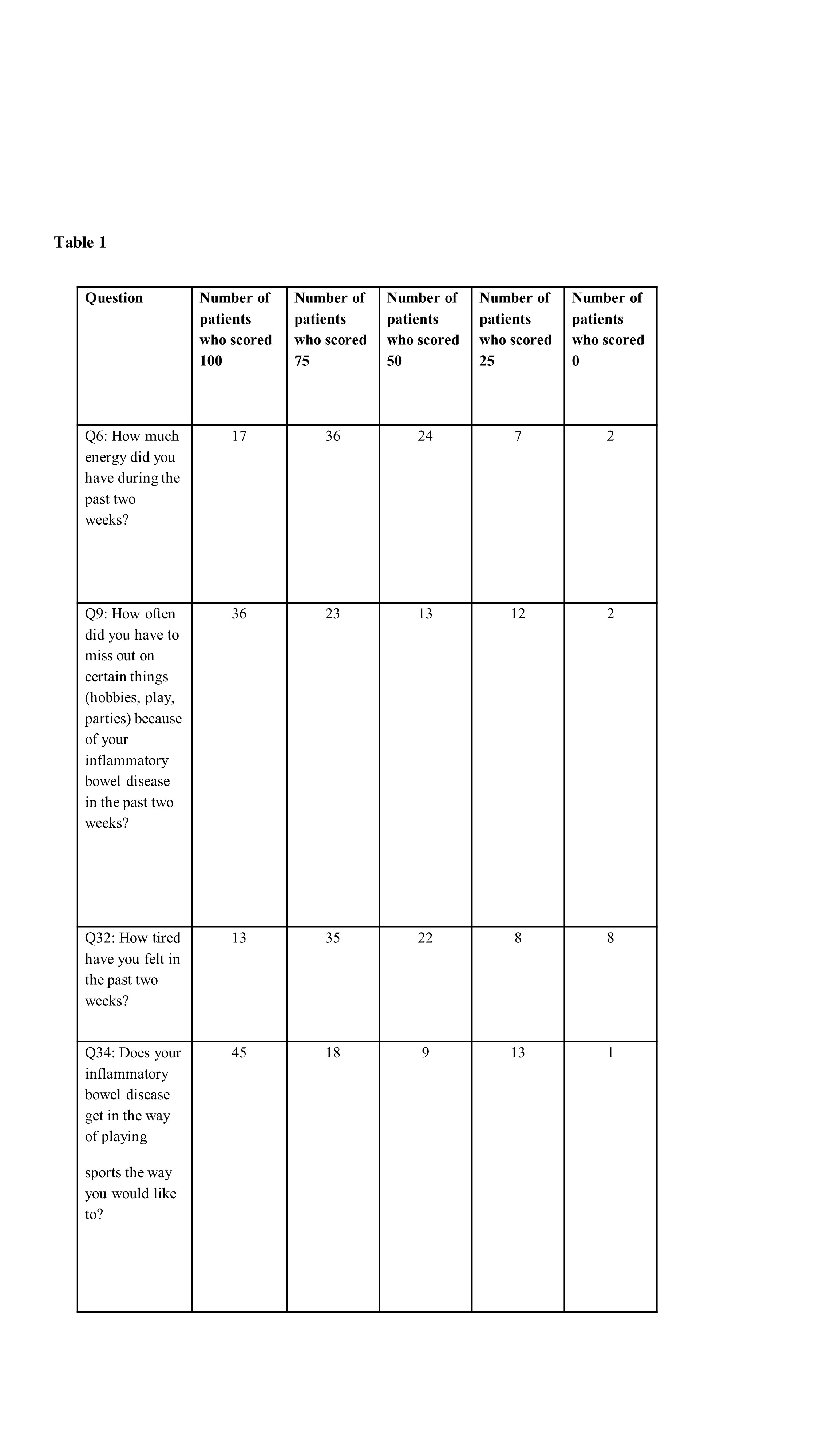
Conclusion
The reporting and assessment of fatigue among pediatric IBD literature is extremely limited. This study is the first to report fatigue rate in children. We show that after induction treatment fatigue rate of any severity may be as high as 53%, moderate fatigue as 33% and severe fatigue as 14%. Fatigue was common also in those in clinical remission. Our results call for a more standardized tool of measuring fatigue in PIBD since its quantification can facilitate a more methodical management of this disabling and underreported symptom.


