P616 IBD reference and biosimilar adalimumab CroSS over study (iBaSS): design considerations and methodology
D. Young1, U. Freudensprung2, C. Harris1, R. Harris1, J. Harvey3, J. Addison4, S. Latter5, F. Cummings1
1University Hospital Southampton NHS Foundation Trust, Department of Gastroenterology, Southampton, UK, 2Biogen International GmbH, n/a, Baar, Switzerland, 3University of Stellenbosch, Department of Statistics and Actuarial Science, Stellenbosch, South Africa, 4Biogen Idec, n/a, Maidenhead, UK, 5University of Southampton, School of Health Sciences, Southampton, UK
Background
Biosimilars of adalimumab (ADA) have been available in Europe since October 2018 and have the potential to generate significant cost savings. SB5 is approved in the EU as an ADA biosimilar, having demonstrated bioequivalence and similar efficacy, safety and immunogenicity to the originator molecule (Humira™) in randomised controlled trials. Given a large number of biosimilar ADA products, it is likely that patients will be asked to switch between multiple ADA products in the future. There is a paucity of published evidence on the clinical outcomes of transitions from reference to biosimilar ADA in the heterogeneous real-world populations encountered in routine clinical practice. A better understanding of these, as well as patients’ related priorities and concerns, is essential to instil confidence in healthcare professionals and patients around such transitions and to improve the transition process.
Methods
iBaSS is a phase IV single-centre, prospective, randomised, single-blind, cross-over study in adult subjects with Crohn’s disease (CD). A cross-over design was selected as it allows each patient to experience both treatments as well as a transition to a biosimilar while remaining on active treatment throughout. This pragmatic study mimics the optimal care pathway of patients transitioning to a biosimilar and enrols a representative population of patients with CD in remission or with mild disease activity. Approximately 150 patients will be exposed in two treatment periods of 24 weeks to ADA biosimilar and reference, with half experiencing two transitions over the study period.
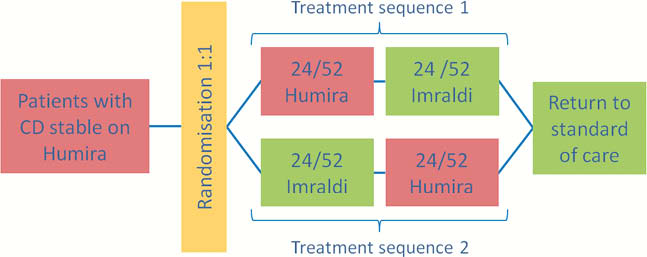
The primary outcome measure is defined as the proportion of patients who maintain the baseline clinical status during both treatment periods as assessed by modified Harvey–Bradshaw Index and IBD-Control. The analysis will be based on a random-effects logistic regression model. Secondary outcome measurements include describing changes in inflammatory markers, immunogenicity and patient experience (captured using semi-structured interviews, Treatment Satisfaction Questionnaire for Medication and a visual analogue scale to assess injection site experience).
Results
Recruitment started in July 2019 with 56 patients recruited so far. The summary baseline characteristics are: 46% male, mean (SD) age at enrolment 42.9 years (14.5) and 84% in remission (mHBI score < 5). Three patients have withdrawn to date (2 withdrew consent and 1 is lost to follow-up).
Conclusion
This describes an achievable study design for a randomised equivalence study of biosimilar transition in patients with Crohn’s disease.


