P629 Long-term effectiveness and safety of ustekinumab (UST) in patients with active Crohn’s disease (CD) in real life: Interim analysis of the SUSTAIN study
M. Chaparro1, S. Sulleiro2, I. Bastón-Rey3, C. Rodríguez4, I. García-Tercero5, P. Ramírez6, S. García-López7, M. Rojas-Feria8, A. Gutiérrez9, J.M. Huguet Malavés10, M.F. García-Sepulcre11, B. Sicilia12, F. Bermejo13, F. Rodríguez-Moranta14, F. Argüelles15, I. Marín16, E. Leo17, M. Arroyo18, M.J. García19, J.M. Vázquez20, D. Ginard21, J. Martínez Cadilla22, C. Rubín de Célix1, A. García-Herola23, A. Hernández-Camba24, M.D. Martín-Arranz25, S. Riestra26, P. Varela27, B. Velayos28, D. Busquets29, C. Dueñas30, E. Fernández-Salgado31, P. Martínez-Montiel32, M.T. Diz-Lois33, Y. González-Lama34, A. Muñagorri35, M. Navarro-Llavat36, C. Guisado2, M. Barreiro-de Acosta3, J.P. Gisbert1, on behalf of SUSTAIN Study Group
1Hospital Universitario de La Princesa, Instituto de Investigación Sanitaria Princesa IIS-IP- Universidad Autónoma de Madrid, and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas CIBERehd, Gastroenterology Unit, Madrid, Spain, 2Janssen, Medical Department, Madrid, Spain, 3Complejo Hospitalario Universitario de Santiago, Gastroenterology Unit, Santiago de Compostela, Spain, 4Complejo Hospitalario de Navarra, Gastroenterology Unit, Pamplona, Spain, 5Hospital Universitario Santa Lucía, Gastroenterology Unit, Cartagena, Spain, 6Hospital Universitario Áraba, Gastroenterology Unit, Vitoria, Spain, 7Hospital Universitario Miguel Servet, Gastroenterology Unit, Zaragoza, Spain, 8Hospital Universitario Nuestra Señora de Valme, Gastroenterology Unit, Sevilla, Spain, 9Hospital General Universitario de Alicante and CIBERehd, Gastroenterology Unit, Alicante, Spain, 10Hospital General Universitario de Valencia, Gastroenterology Unit, Valencia, Spain, 11Hospital General Universitario de Elche, Gastroenterology Unit, Elche, Spain, 12Complejo Asistencial Universitario de Burgos, Gastroenterology Unit, Burgos, Spain, 13Hospital Universitario de Fuenlabrada- Instituto de Investigación de Hospital La Paz IdiPaz- Madrid, Gastroenterolgoy Unit, Madrid, Spain, 14Hospital Universitario de Bellvitge, Gastroenterology Unit, Barcelona, Spain, 15Hospital Universitario Virgen Macarena, Gastroenterology Unit, Sevilla, Spain, 16Hospital Universitario Gregorio Marañón- IiSGM and CIBERehd, Gastroenterology Unit, Madrid, Spain, 17Hospital Universitario Virgen del Rocío, Gastroenterology Unit, Sevilla, Spain, 18Hospital Clínico Universitario, Lozano Blesa and CIBERehd- IIS Aragón, Gastroenterology Unit, Zaragoza, Spain, 19Hospital Universitario Marqués de Valdecilla, Gastroenterology Unit, Santander, Spain, 20Hospital Juan Ramón Jiménez, Gastroenterology Unit, Huelva, Spain, 21Hospital Universitario Son Espases, Gastroenterology Unit, Palma de Mallorca, Spain, 22Hospital Alvaro Cunqueiro, Gastroenterology Unit, Vigo, Spain, 23Hospital Marina Baixa, Gastroenterology Unit, Alicante, Spain, 24Hospital Universitario Nuestra Señora de la Candelaria, Gastroenterology Unit, Santa Cruz de Tenerife, Spain, 25Hospital Universitario La Paz-Innate Immunity Group- IdiPAZ Institute for Health Research, Gastroenterology Unit, Madrid, Spain, 26Hospital Universitario Central de Asturias, Gastroenterology Unit, Oviedo, Spain, 27Hospital Universitario de Cabueñes, Gastroenterology Unit, Gijón, Spain, 28Gastroenterology Unit, Hospital Clínico Universitario de Valladolid, Valladolid, Spain, 29Hospital Universitari de Girona Dr. Josep Trueta, Gastroenterology Unit., Girona, Spain, 30Hospital San Pedro de Alcántara, Gastroenterology Unit, Cáceres, Spain, 31Complexo Hospitalario Universitario de Pontevedra, Gastroenterology Unit, Pontevedra, Spain, 32Hospital Universitario Doce de Octubre, Gastroenterology Unit, Madrid, Spain, 33Complejo Hospitalario Universitario de A Coruña, Gastroenterology Unit, A Coruña, Spain, 34Hospital Universitario Puerta de Hierro Majadahonda, Gastroenterology Unit, Madrid, Spain, 35Hospital Universitario Donostia, Gastroenterology Unit, San Sebastián, Spain, 36Hospital de Sant Joan Despí Moisès Broggi, Gastroenterology Unit, Barcelona, Spain
Background
Post-marketing data are required to confirm the durability and the long-term benefit and safety of UST in CD in clinical practice. Our aims were: (1) to evaluate the retention rate of UST in CD patients and to identify predictive factors of UST discontinuation; (2) to assess UST short-term effectiveness; (3) to analyse the durability of the response to UST in the long-term; and (4) to evaluate the safety of UST in clinical practice.
Methods
Retrospective, multicentre study (>60 centres). Patients with active CD [(Harvey–Bradshaw (HBI) >4)] that received at least one dose of UST intravenously before July 2018 were included. Clinical activity plus biochemical parameters were assessed at every UST administration. Clinical remission was defined as HBI score ≤4, and clinical response as a decrease in HBI ≥3 points. Loss of efficacy was defined as reappearance of symptoms that led to intensify the treatment dose, add another medication to control CD, switching or surgery in patients with short-term remission. The retention rate of UST treatment and the cumulative incidence of loss of efficacy were evaluated by survival curves, and predictive factors were assessed by Cox-regression. The short-term response was evaluated at week 8 and after the induction (week 16). Factors associated with short-term remission were assessed by multivariate analysis. Adverse events were recorded. Data quality was assured by remote monitoring.
Results
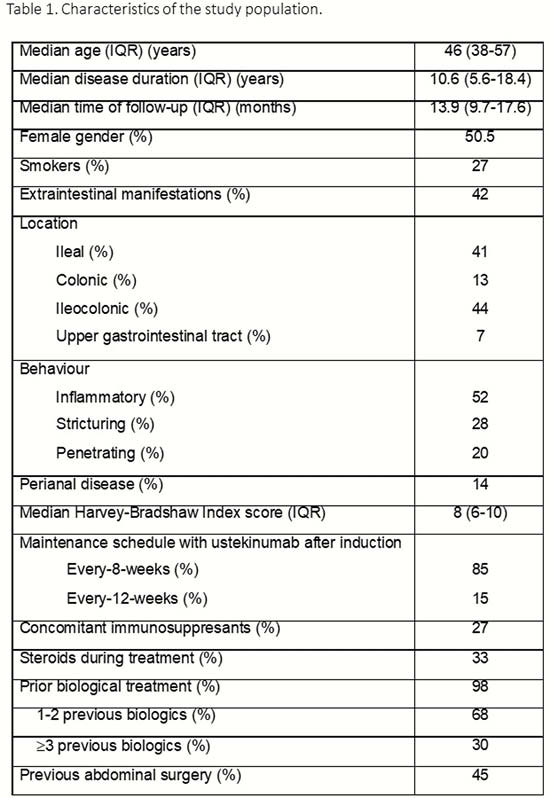
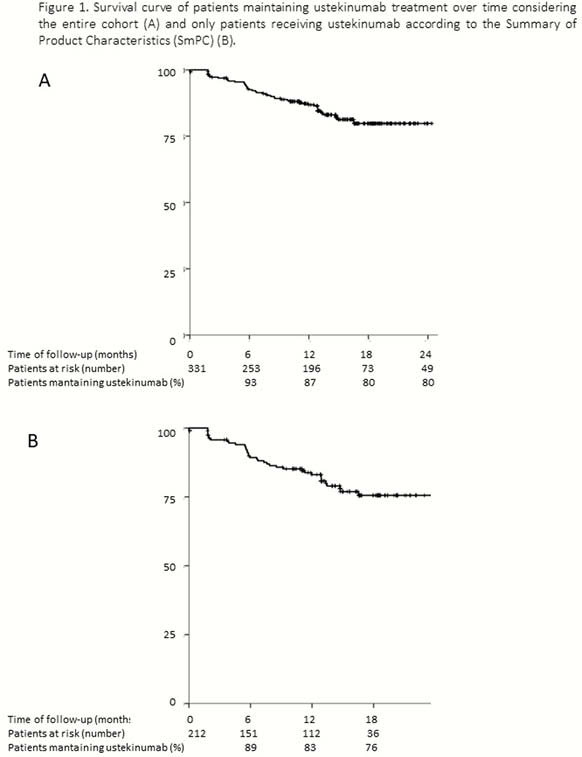
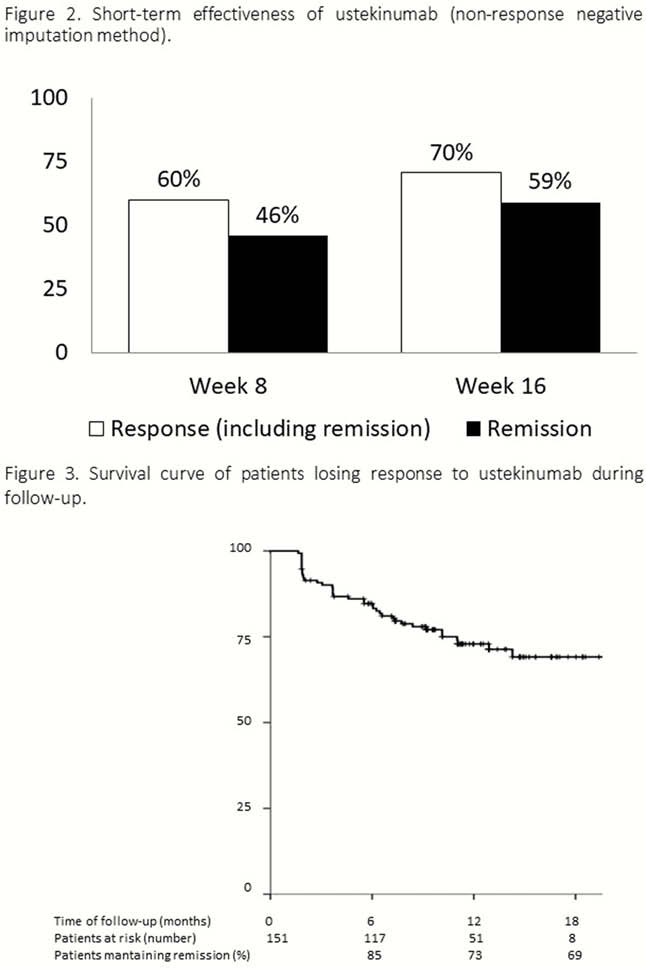
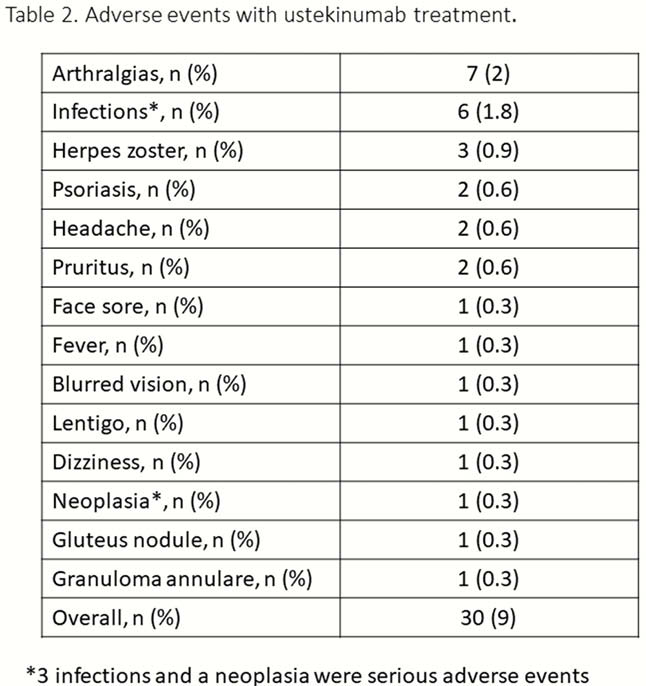
331 CD patients have been included up to date (Table 1). The incidence rate of UST discontinuation was 15% per patient-year of follow-up: 8%, 13% and 20% at 6, 12 and 18 months (Figure 1). Previous surgery was the only factor associated with a higher risk of UST discontinuation [Hazard ratio (HR) = 2.03, 95% confidence interval (CI) = 1.1–3.6]. Short-term efficacy is shown in Figure 2. Previous surgery (OR = 0.3, 95% CI = 0.2–0.6) and higher HBI score at baseline (OR = 0.8, 95% CI=0.8–0.9) were associated with an impaired response to UST at week 16. The cumulative incidence of loss of response was 32% per-patient-year of follow-up (Figure 3); A higher HBI score at baseline was associated with a higher risk of losing response (HR = 1.2, 95% CI = 1.1–1.3). Neither the concomitant treatment with immunosuppressants nor the number of previous biologics were associated with UST short- and long-term benefit. Thirty adverse events were reported in 25 (7%) patients (Table 2).
Conclusion
Sustain is the largest real clinical practice study of UST to treat CD patients with the longest follow-up reported to date. UST was demonstrated to be effective in real-world use in the short and long run. Safety was consistent with the known profile of UST.


