P634 Predictors of response and course in patients with inflammatory bowel disease treated with biological therapy: The Danish IBD-Biobank project
M. Zhao1, F. Bendtsen1, A.M. Petersen1,2, L. Larsen3, T. Jess3,4, A. Dige5, C. Hvas5, J. Seidelin6, J. Burisch1
1Hvidovre University Hospital, Gastro Unit- Medical Division, Hvidovre, Denmark, 2Hvidovre University Hospital, Dept. of Clinical Microbiology, Hvidovre, Denmark, 3Aalborg University Hospital, Department of Gastroenterology and Hepatology, Aalborg, Denmark, 4The State Serum Institute, Departmentof Epidemiology Research, Copenhagen, Denmark, 5Aarhus University Hospital, Department of Hepatology and Gastroenterology, Aarhus, Denmark, 6Herlev University Hospital, Gastro Unit- Medical Division, Herlev, Denmark
Background
Biologic treatment has revolutionised the treatment of inflammatory bowel diseases (IBD) and has been shown to reduce surgery and hospitalisation rates in patients with severe disease. Up to one-third of patients do not respond to first-line biologic drugs and another third loses response with time. This highlights an unmet need for optimising the use of biologics and for predicting treatment response to biologics. We present the initial recruitment stages and experimental pipeline for The Danish IBD Biobank which aims to identify predictive biomarkers of response and disease course in patients with IBD who receive biologics.
Methods
The Danish IBD Biobank constitutes a multicenter prospective cohort study aiming to include 840 biological-naïve adult patients with IBD who initiate biologic therapy in a three-year period from May 2019 and onwards. Primary outcomes are the occurrence of primary non-response (PNR) evaluated at week 14–16 and loss of response (LOR) evaluated during the entire follow-up. Patients will be followed with clinical data for at least one year or until May 2022 and sampled for blood, stool and intestinal tissue during the first year of treatment. Blood and stool samples are collected at each visit for biologic treatment and upon change in drug type, dosing or discontinuation. Intestinal tissue is sampled whenever an endoscopy is performed and after one year of treatment (Figure 1).
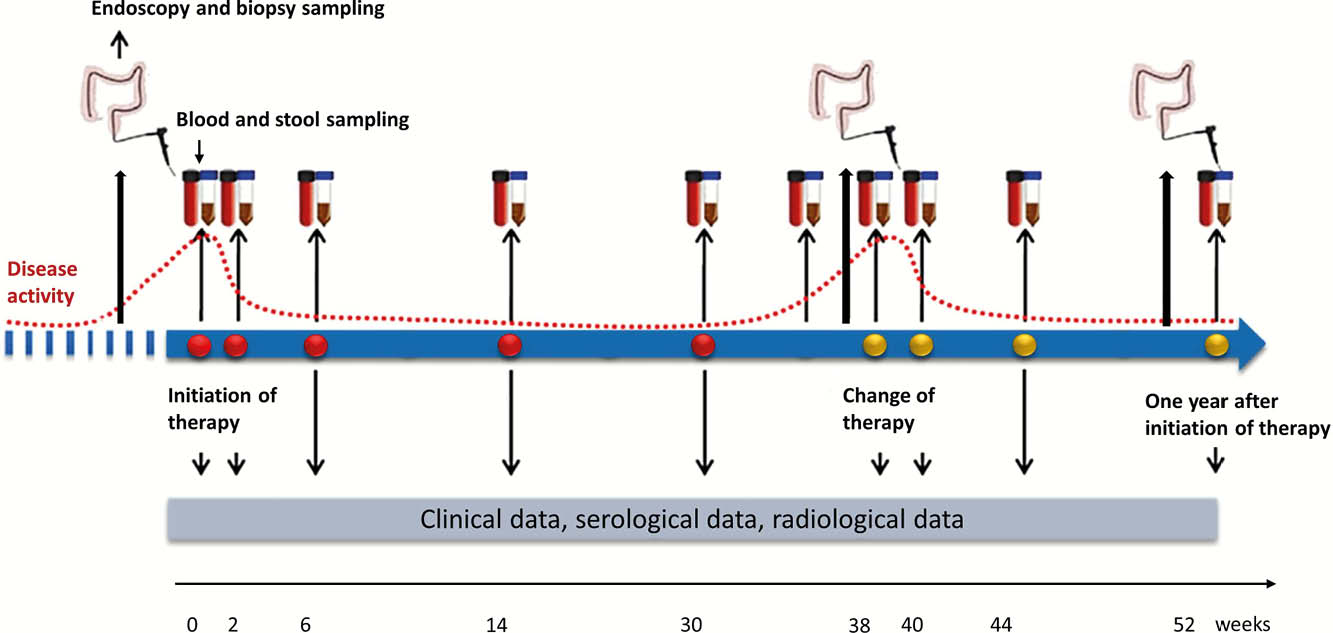
Results
As of 15 November 2019, 132 patients have been recruited to the cohort. Biologic treatment was indicated due to acute severe ulcerative colitis (UC) or chronic active UC in 13% and 43% of patients, respectively, while 38% and 6% received treatment for luminal Crohn’s disease (CD) and perianal CD. In accordance with national guidelines issued by the Danish Medicines Council, infliximab was the most commonly used first-line drug (98%), followed by vedolizumab (2%) and adalimumab (0.8%). Among 68 patients who have completed induction therapy so far, PNR occurred in 35% (62% UC, 38% CD). Among those with PNR, 50% switched class, 21% discontinued treatment and 29% underwent surgery. Blood and stool samples and intestinal biopsies are stored in a biobank awaiting characterisation of RNA and protein expression using multiplex immunoassay, 16S and Illumina sequencing, and characterisation of mucosal gene and protein expression (Figure 2).
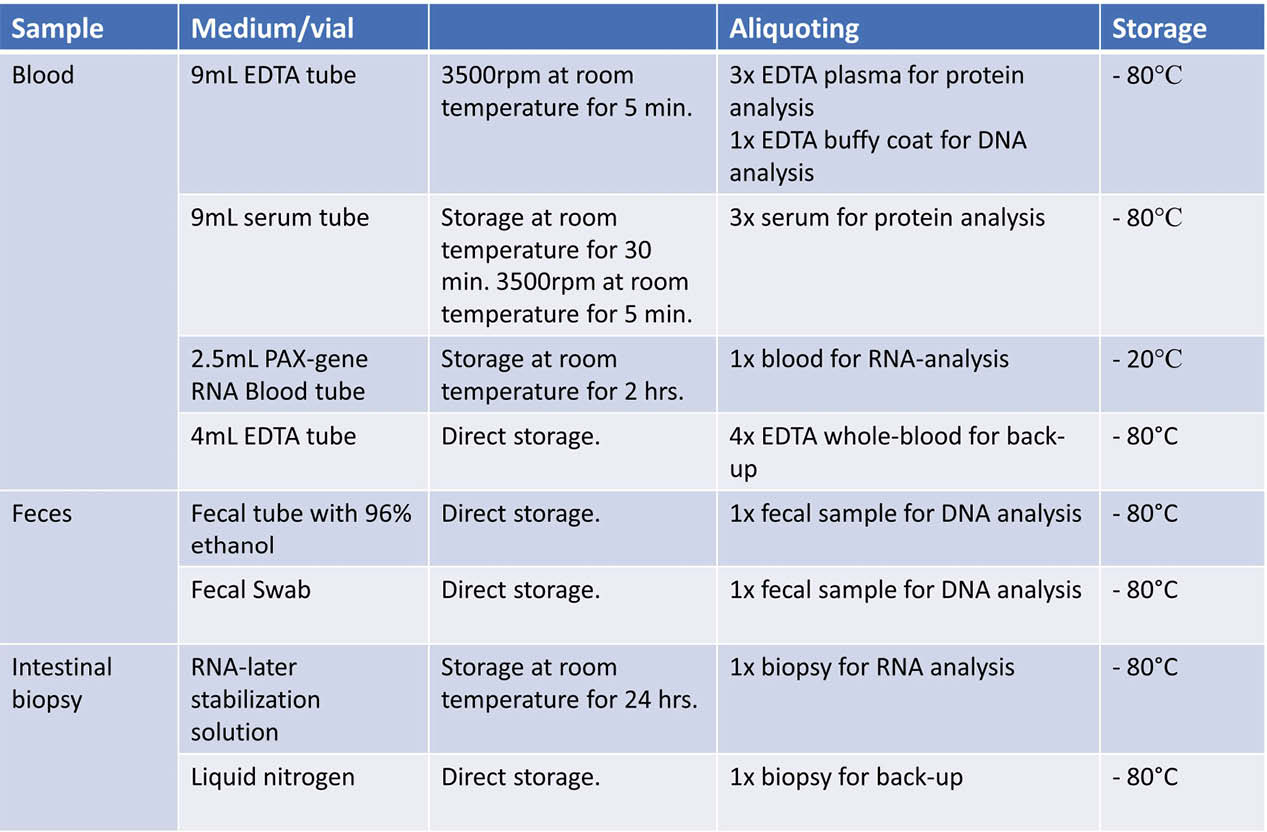
Conclusion
The Danish IBD Biobank project aiming to identify predictive transcriptomic, microbial and proteomic biomarkers associated with treatment response and outcomes to biological therapy in a prospective cohort of biological-naïve IBD patients has been successfully launched.
This study is funded by an unrestricted grant from Takeda A/S and public fund hosted by Hvidovre Hospital.


