P635 Superior outcomes with early biologic-combination treatment in Crohn’s Disease: Data from an international inception cohort
M. Matheeuwsen1, K. Vermeijden1, P. Bossuyt2, L. Pouillon2, F. Baert3, N. de Boer4, M. Duijvestein4, M. Löwenberg1, C. Ponsioen1, W. Bemelman5, G. D’Haens1, K. Gecse1
1Amsterdam UMC- Academic Medical Center, Department of Gastroenterology and Hepatology, Amsterdam, The Netherlands, 2Imelda GI Clinical Research Center, Department of Gastroenterology, Bonheiden, Belgium, 3AZ Delta hospital, Department of Gastroenterology, Roeselare, Belgium, 4Amsterdam UMC- VU University Medical Center- AGandM Research Institute, Department of Gastroenterology and Hepatology, Amsterdam, The Netherlands, 5Amsterdam UMC- Academic Medical Center, Department of Surgery, Amsterdam, The Netherlands
Background
Crohn’s disease (CD) is characterised by intestinal inflammation leading to progressive bowel damage. Over the past years, treatment goals have evolved from clinical remission to prevention of structural bowel damage. It is currently unknown which treatment options and sequences lead to superior outcomes in patients with newly diagnosed CD.
Methods
The aim of this study was to compare different treatment strategies and associated outcomes in newly CD. We performed a retrospective study in adult patients diagnosed with CD in Amsterdam (The Netherlands), Bonheiden or Roeselare (Belgium) between 2009 and 2019. Based on first-line treatment choice, patients were assigned to one of the five pre-defined treatment strategies. The primary outcome was time to treatment failure, defined as a switch to another CD treatment, escalating CD treatment, need for endoscopic balloon dilation or CD-related surgery, CD-related hospitalisation or a new perianal fistula. Time to treatment failure was compared between different treatment groups using Kaplan–Meier analysis and Cox proportional hazard models.
Results
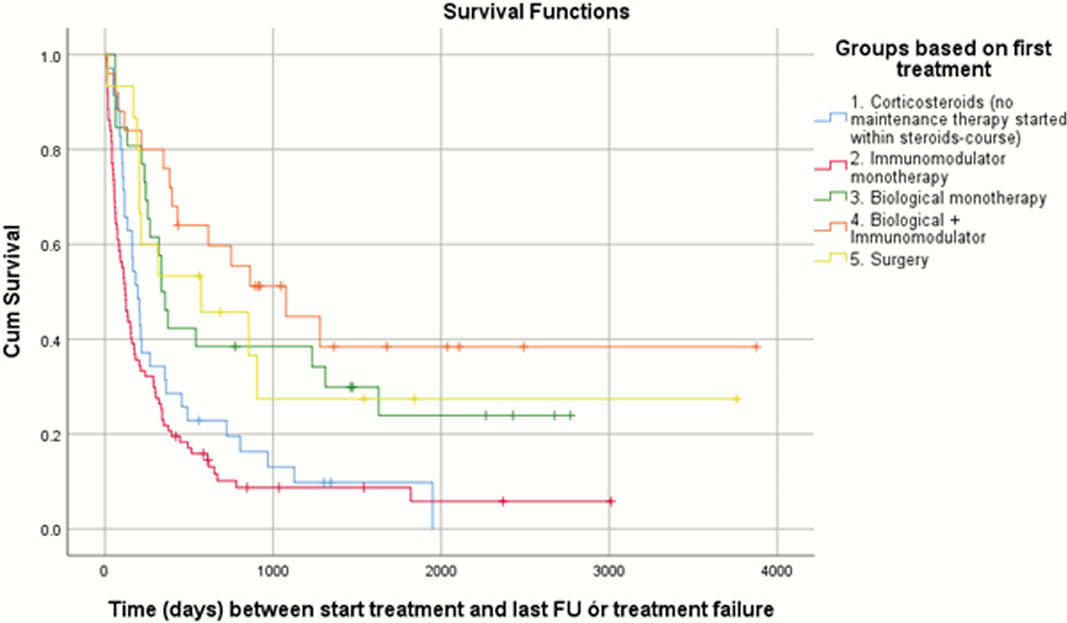
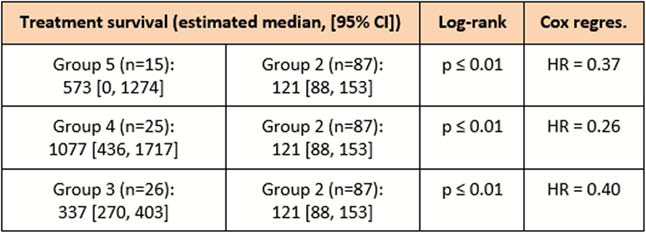
In total, 188 newly diagnosed patients were included. Treatment with corticosteroids only as first-line treatment (group 1) was started in 35 patients, immunomodulators (azathioprine, 6-mercaptopurine or methotrexate; group 2) in 87 patients, biological therapy (infliximab, adalimumab or vedolizumab; group 3) in 26 patients, and biological therapy in combination with an immunomodulator (group 4) in 25 patients. Fifteen patients underwent direct surgery (group 5). Baseline characteristics (C-reactive protein, CD Endoscopic Index of Severity, Simple Endoscopic Score for CD, smoking status) were similar between the different treatment groups, although group 5 had significantly more patients with stricturing (Montreal B2) and penetrating (Montreal B3) disease subtypes [Table1]. Log-rank test revealed significantly longer median time to treatment failure in group 5 (573 days; 95% confidence interval (CI) 0, 1274; hazard ratio (HR) 0.37), group 4 (1077 days; 95% CI 436, 1717; HR 0.26) and group 3 (337 days; 95% CI 270, 403; HR 0.40) compared with group 2 (121 days; 95% CI 88, 153) (
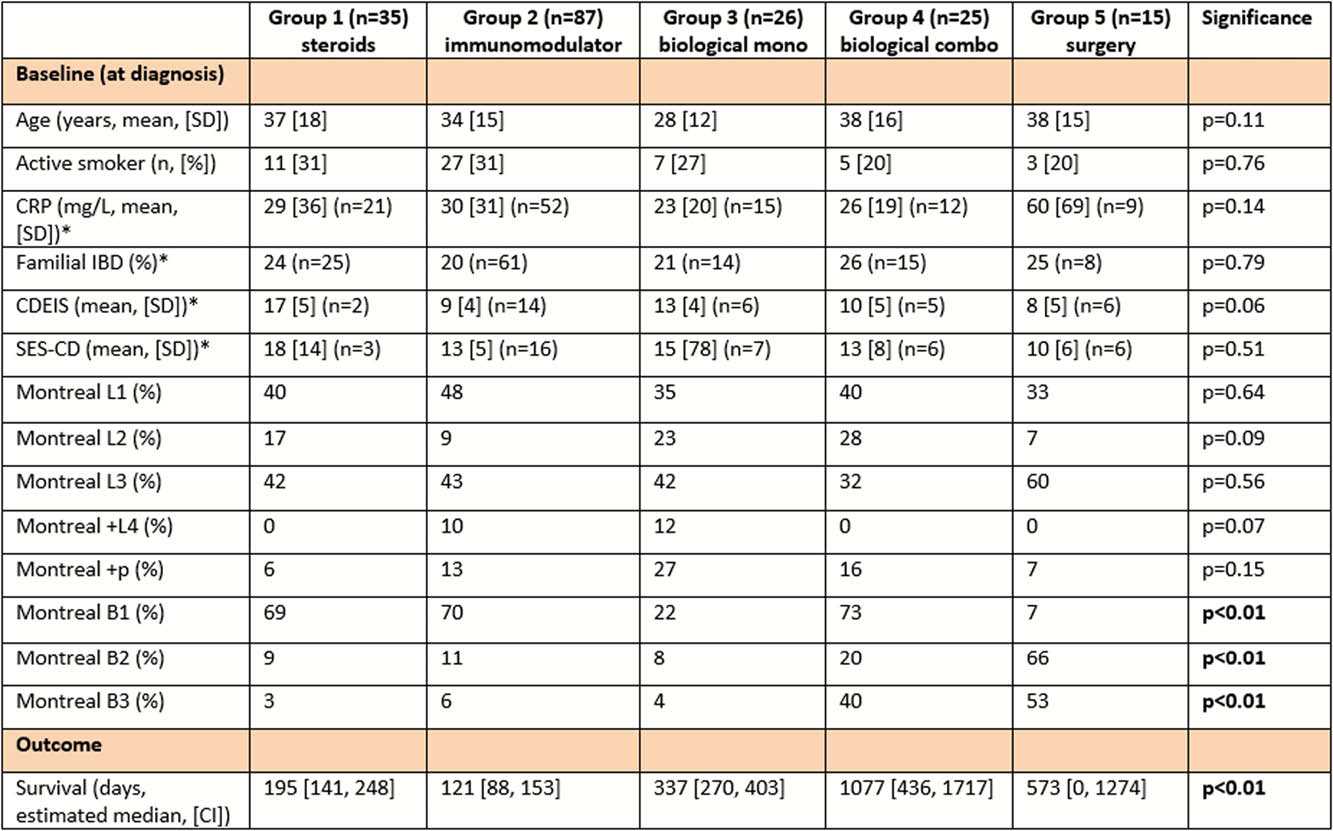
CD Endoscopic Index of Severity (CDEIS), Simple Endoscopic Score for CD (SES-CD), *based on
Conclusion
These real-world data seem to show superior treatment survival in newly diagnosed CD patients receiving first-line treatment with biologicals (as mono- and combination therapy) and early surgery compared with patients receiving first-line treatment with immunomodulators alone.


