P653 Efficacy and safety of an additional 8 weeks of tofacitinib induction therapy: Updated results of the OCTAVE Open study for tofacitinib 8-week induction non-responders
D.T. Rubin1, M.C. Dubinsky2, S. Danese3, R. Saad-Hossne4, D. Ponce de Leon5, E. Maller6, N. Lawendy6, K. Kwok7, C. Su6
1Inflammatory Bowel Disease Center, University of Chicago Medicine, Chicago, Illinois, USA, 2Department of Pediatrics and Medicine- Icahn School of Medicine at Mount Sinai, New York, New York, USA, 3IBD Center- Department of Gastroenterology- Humanitas Research Hospital, Rozzano, Milan, Italy, 4IBD Group- Botucatu Medical School- São Paulo State University, São Paulo, Brazil, Brazil, 5Pfizer Inc., Lima, Peru, Peru, 6Pfizer Inc., Collegeville, Pennsylvania, USA, 7Pfizer Inc., New York, New York, USA
Background
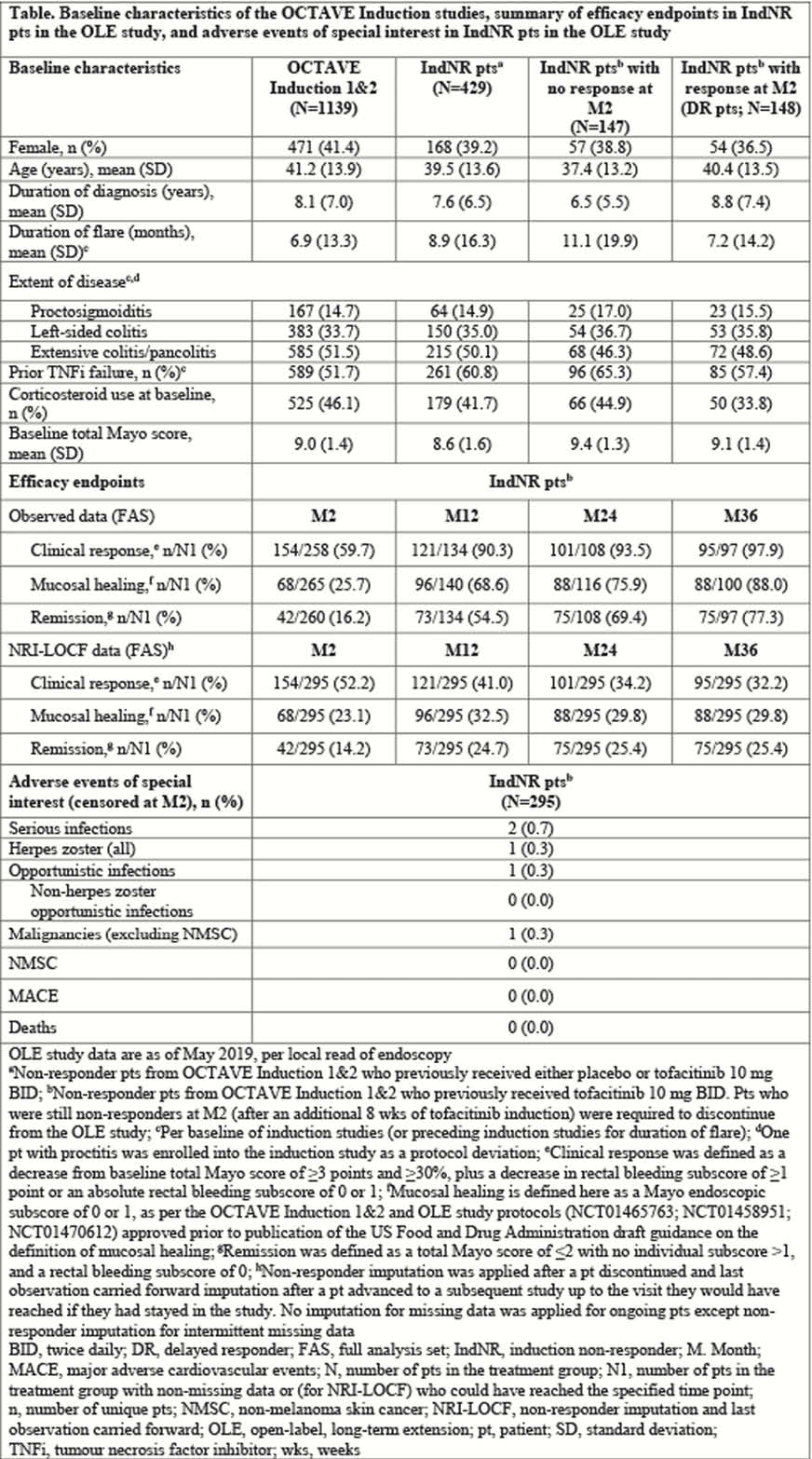
Tofacitinib is an oral, small-molecule JAK inhibitor for the treatment of ulcerative colitis (UC). Efficacy and safety of tofacitinib were demonstrated in three Phase 3, randomised, placebo-controlled trials in patients with moderate to severe UC.1 Patients who received tofacitinib 10 mg twice daily (BID) for 8 weeks (weeks) in OCTAVE Induction 1 and 2 (NCT01465763; NCT01458951) and did not achieve a clinical response (ie induction non-responders [IndNR]) could enter an ongoing, Phase 3, open-label, long-term extension (OLE) study (OCTAVE Open; NCT01470612). We present an update, as of May 2019, of previously published data up to December 2016 from the OLE study for IndNR patients.2
Methods
IndNR patients received tofacitinib 10 mg BID in the OLE study. Patients who were still non-responders after an additional 8 weeks of induction were required to discontinue. Clinical response, remission and mucosal healing were evaluated up to Month (M)36 of the OLE study. Adverse events (AEs) and serious AEs (SAEs) are also presented.
Results
429 IndNR patients enrolled in the OLE study, 295 of which received tofacitinib 10 mg BID during induction trials (Table). The proportions of patients with prior tumour necrosis factor inhibitor and baseline corticosteroid use were slightly higher in non-responders than responders at M2; other baseline characteristics were generally similar in responders and non-responders. At M2, 59.7%, 25.7% and 16.2% of patients achieved clinical response, mucosal healing and remission, respectively (as observed). Corresponding non-responder imputation and last observation carried forward values were 52.2%, 23.1% and 14.2% (Table). The table shows data up to M36. The proportions of patients with AEs, SAEs and discontinuations due to AEs for IndNR patients censored at M2 (52.2%, 3.7% and 2.4%, respectively) were similar to those for all patients in OCTAVE Induction 1 and 2 (tofacitinib: 55.4%, 3.8% and 3.9%; placebo: 56.4%, 6.0% and 4.3%), with no new safety risks identified (table).
Conclusion
The majority of patients who did not achieve clinical response to tofacitinib 10 mg BID for 8 weeks in the induction studies – and subsequently received an additional 8 weeks of tofacitinib 10 mg BID in the OLE study—achieved clinical response, with a considerable number of patients in remission and/or mucosal healing at M36. No new safety risks were observed in the additional 8 weeks of induction. These data support extended induction with an additional 8 weeks of tofacitinib for non-responders to 8-week induction. Current product labelling recommends 5 mg BID for maintenance in responders.3,4
Sandborn WJ
Feagan BG
EMA. SmPC: Xeljanz. www.ema.europa.eu
FDA. PI: Xeljanz. www.pfizer.com


