P654 Influence of the interval of time between anti-TNF and vedolizumab or ustekinumab in the response to treatment in patients with inflammatory bowel disease
I. Bastón Rey1, M. Costa1, C. Calviño-Suarez1, V. Mauriz-Barreiro1, D. De la Iglesia1, J. Gonzalez2, R. Ferreiro-Iglesias1, J.E. Dominguez- Munoz1, M. Barreiro-de Acosta1
1University Hospital Santiago De Compostela CHUS, Department of Gastroenterology- IBD Unit, Santiago De Compostela, Spain, 2University Hospital Santiago De Compostela CHUS, Department of Pharmacy, Santiago De Compostela, Spain
Background
There is no evidence about the time that it is reasonable to wait until starting Vedolizumab (VDZ) or Ustekinumab (UST) in case of loss of response to anti-TNF therapy. The aim of our study was to evaluate whether the time between the change from any anti-TNF drug to VDZ or UST had an influence on the risk of treatment failure and the rate of adverse events or severe infections.
Methods
A retrospective, observational single-centre study was designed. Inclusion criteria were all adult patients with moderate-to-severe IBD who started treatment with VDZ or UST due to loss of response to anti-TNF (Infliximab or Adalimumab). Exclusion criteria were patients who had received treatment for different indications such as prevention of recurrence or extra-intestinal manifestations. Patients that changed directly from VDZ to UST or from UST to VDZ were also excluded. We defined two groups of periods based on the interval of time between the last dose of anti-TNF and the first dose of VDZ or UST: Group A: early (≤30 days), Group B: late (31–60 days). Patients that had a waiting time of over 60 days were also excluded. Treatment failure after the initiation of VDZ or UST was defined as the need for dose intensification, surgical resection, therapy removal for ineffectiveness or adverse reaction. The existence of infections was also evaluated. Results are shown as percentages, median, range and Hazard Ratio (CI 95%). Fisher test and Cox Regression analysis were also performed.
Results
46 patients (45.6% CD) were consecutively included (mean age 47.6 years). 17 had initially been treated with Infliximab and 29 with Adalimumab. Thirty patients were changed to VDZ and 16 to UST. Twenty-three patients were included in Group A and 23 in Group B. Treatment failure was observed in 20 patients: 9/23 in group A and 11/23 in group B with a mean follow-up of 323 days (range 152–432). The duration of the interval of time between stopping anti-TNF and starting VDZ or UST was not associated with increased risk of treatment failure (
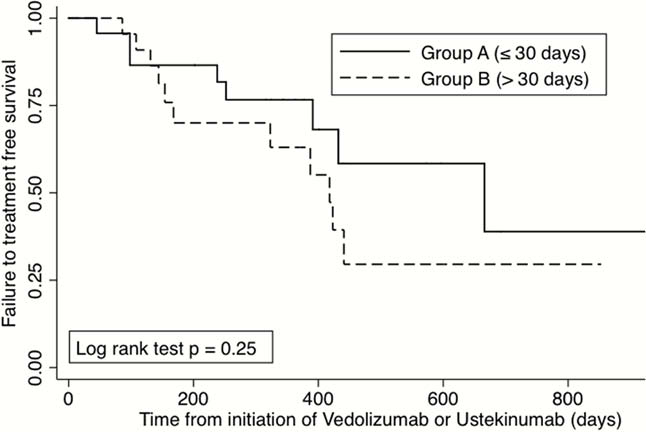
Conclusion
The interval of time between anti-TNF and VDZ or UST had no influence on the rates of treatment failure, adverse events or infections in our cohort.


