P655 Pharmacokinetics of ustekinumab in children and adolescents with moderately to severely active Crohn’s disease: Results from UniStar, a phase 1 study
O. Adedokun1, J. Hyams2, D. Turner3, A. Griffiths4, N. Terry5, L. Padgett5, D. Jacobstein6, C. O’Brien5, J. Rosh7
1Janssen Research and Development- LLC, Clinical Pharmacology, Horsham- PA, USA, 2Connecticut Children’s Medical Center, Pediatrics, Hartford- CT, USA, 3Shaare Zedek Medical Center, Pediatrics, Jerusalem, Israel, 4SickKids Hospital, Pediatrics, Toronto, ON, Canada, 5Janssen Research and Development, LLC, Research and Development, Horsham, PA, USA, 6former employee of Janssen Research and Development, LLC, current employee of Provention Bio, Research and Development, Horsham, PA, USA, 7Goryeb Children’s Hospital/Atlantic Health, Pediatrics, Morristown, NJ, USA
Background
Ustekinumab (UST) is approved for the treatment of adults with moderate to severe Crohn’s disease (CD) or ulcerative colitis. In the UniStar study, the pharmacokinetics (PK) of UST and its relationship with efficacy was evaluated in children who failed prior therapy. UniStar consisted of a PK portion (week 0–16) and extension (week 16–216); we report data through week 16.
Methods
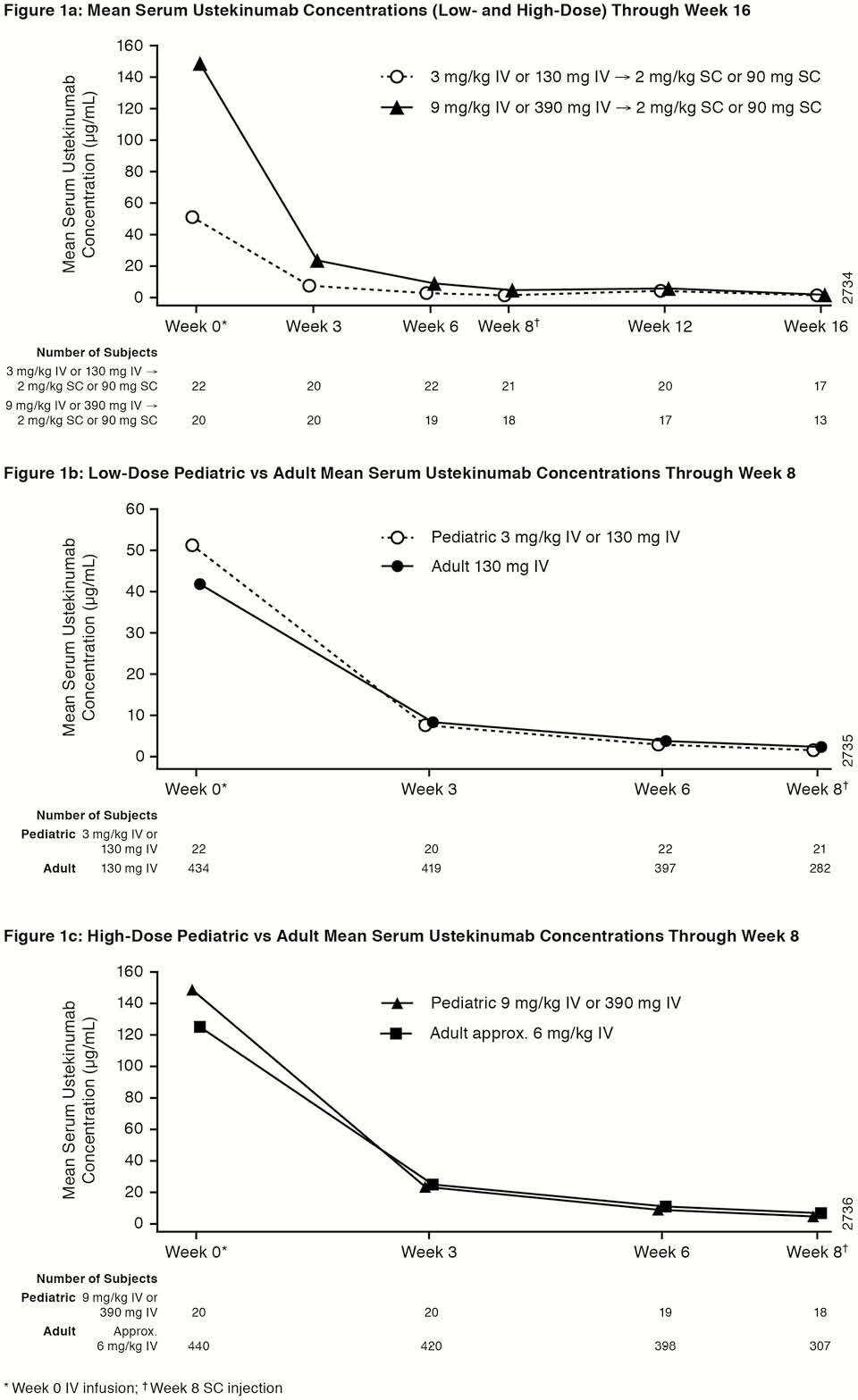
UniStar is a multicentre, double-blind study (NCT02968108) designed to assess the PK, safety, and efficacy of UST in children (2–<18 years) with moderately to severely active CD, Paediatric CD Activity Index (PCDAI) score >30, and evidence of inflammation as measured by C-reactive protein >3.0 mg/l or faecal calprotectin >250 µg/g or ulcerations on ileocolonoscopy despite adequate treatment with corticosteroids and/or immunomodulators and/or anti-TNF therapies. Patients were randomised (1:1) and stratified by body weight (BW) and prior anti-TNF use for induction to one of 2 weight range-based IV doses: 130mg vs. 390 mg if BW ≥40 kg and 3mg/kg vs. 9 mg/kg if BW <40 kg. At week 8, all patients received a single subcutaneous (SC) UST maintenance dose of 90mg if BW ≥40 kg or 2 mg/kg if BW <40 kg. UST PK outcomes were assessed and compared with adult Phase 3 CD trials.
Results
44 patients (59% ≥40 kg; >90% anti-TNF exposed) were randomised and treated with UST (
Conclusion
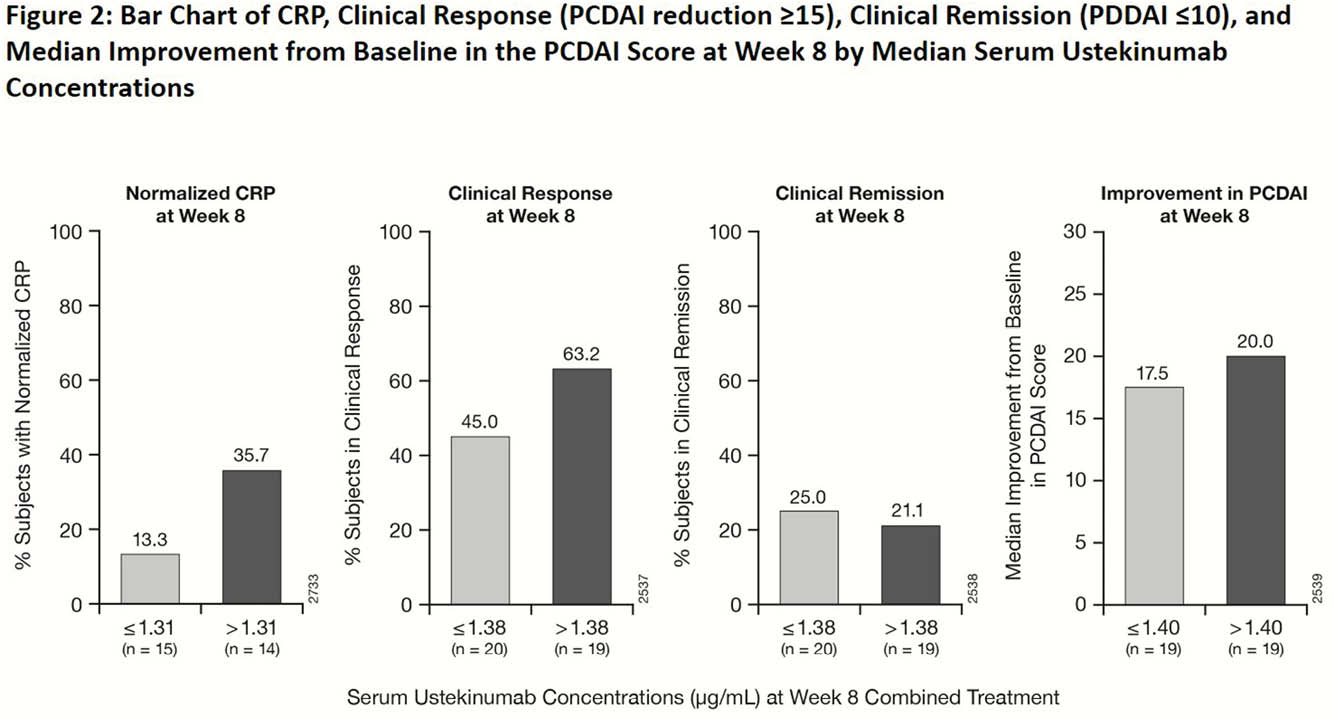
Overall, UST PK was generally comparable between paediatric and adult patients with CD. A trend towards better efficacy outcomes with higher UST concentrations was observed in children similar to adults with CD.


