P658 A pooled analysis of serum phosphate measurements and transient decreased phosphate levels in 45 interventional trials with ferric carboxymaltose
J.M. Stein1, I. Schiefke2, U.M. Göhring3, V. Fabien4, G. Rosano5
1DGD Hospital Frankfurt-Sachsenhausen, Klinik für Gastroenterologie/Onkologie, Frankfurt/Main, Germany, 2Klinikum St. Georg, Department of Gastroenterology, Hepatology, Diabetology and Endocrinology, Leipzig, Germany, 3Vifor Pharma, Clinical Development, Zurich, Switzerland, 4Vifor Pharma, Biometrics, Zurich, Switzerland, 5St George’s Hospitals NHS Trust, Clinical Academic Cardiovascular Group-, London, UK
Background
ECCO guidelines acknowledge the limitations of oral iron for iron deficiency anaemia in IBD and recommend first-line IV iron in patients with active disease. Ferric carboxymaltose (FCM) is approved for iron deficiency when oral iron is ineffective/inappropriate. FCM has shown benefit in RCTs, and as with other IV irons, is known to cause a transient decrease in serum phosphate (PO4), which in most cases is asymptomatic. The aim of this pooled analysis was to characterise the frequency, duration, risk factors, and clinical signs of hypophosphataemia (HP) across 45 interventional trials with FCM.
Methods
15,080 subjects were included (FCM
Results
2,760 FCM subjects (40.1%) with normal PO4 at baseline developed HP (PO4 <2.5 mg/dl) on study. PO4 levels normalised in 1,705 subjects (61.8%); mean time to normalisation (±SD) was 40.7 days (±35.31). PO4 normalisation during follow-up did not occur in 177 subjects (0.06%). Independent risk factors for moderate/severe HP within 12 weeks of treatment included treatment setting (gastroenterology or neurology vs. women’s health), lower baseline ferritin level, maximum single-dose, advanced age, lower weight at baseline (all
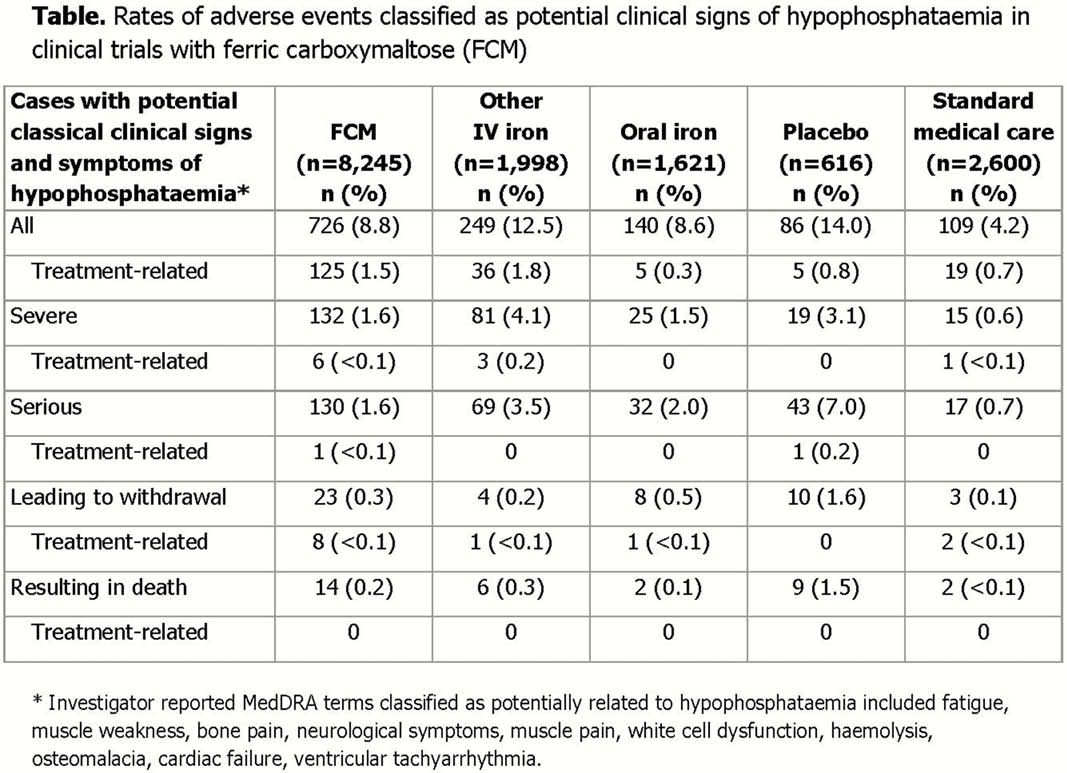
Conclusion
HP is recognised as a common adverse drug reaction with FCM as with several other IV iron preparations. This analysis shows that the transient decreases in PO4 levels were more frequent in patients receiving multiple and/or large doses of FCM but were not associated with an increased rate of potential signs/symptoms of HP.


