P662 Pre-treatment vitamin D concentrations do not predict therapeutic outcome to anti-TNF therapies in biologic-naïve patients with active luminal Crohn’s disease
Lin, S.(1,2)*;Chanchlani, N.(1,2);Smith, R.(1,2);Roberts, C.(1,2);Nice, R.(3);McDonald, T.J.(3);Hamilton, B.(2);Bewshea, C.(2);Kennedy, N.A.(1,2);Goodhand, J.R.(1,2);Ahmad, T.(1,2);
(1)Royal Devon University Healthcare NHS Foundation Trust, Gastroenterology, Exeter, United Kingdom;(2)University of Exeter, Exeter Inflammatory Bowel Disease and Pharmacogenetics Research Group, Exeter, United Kingdom;(3)Royal Devon University Healthcare NHS Foundation Trust, Biochemistry- Exeter Clinical Laboratory International, Exeter, United Kingdom; PANTS consortium
Background
Vitamin D has a regulatory role in innate and adaptive immune processes. Previous small studies have reported that low pre-treatment vitamin D concentrations are associated with primary non-response and shorter drug persistence to anti-TNF therapy. We sought to assess whether pre-treatment 25-hydroxyvitamin D concentrations predicted primary non-response and non-remission to infliximab and adalimumab in patients with Crohn’s disease recruited to the PANTS (Personalised Anti-TNF Therapy in Crohn’s disease) study.
Methods
We used composite endpoints using the Harvey Bradshaw Index (HBI) in adults and the short paediatric Crohn’s disease activity index (sPCDAI) in children, corticosteroid use, and C-reactive protein (CRP) to define primary non-response (Figure 1). Remission was defined as CRP of ≤3 mg/L and HBI of ≤4 points (short paediatric Crohn’s disease activity index ≤15 in children), without corticosteroid therapy or exit for treatment failure. 25-hydroxyvitamin D concentrations were measured in stored baseline serum samples and cut-offs for vitamin D status were: deficiency < 25nmol/L, insufficiency 25-50nmol/L and adequacy/sufficiency > 50nmol/L.
Results
Samples from 659/898 infliximab (526 Remicade; 133 biosimilar CT-P13) and 448/605 adalimumab (448 Humira) treated patients included in the effectiveness analysis of the PANTS study were included. Overall, 17.1% (189/1107; 95% confidence interval [CI] 15.0 - 19.4%) and 47.7% (528/1107; 95% CI 44.8 - 50.6%) patients had vitamin D deficiency and insufficiency, respectively. At baseline, 22.2% (246/1107) patients were receiving some form of vitamin D supplementation.
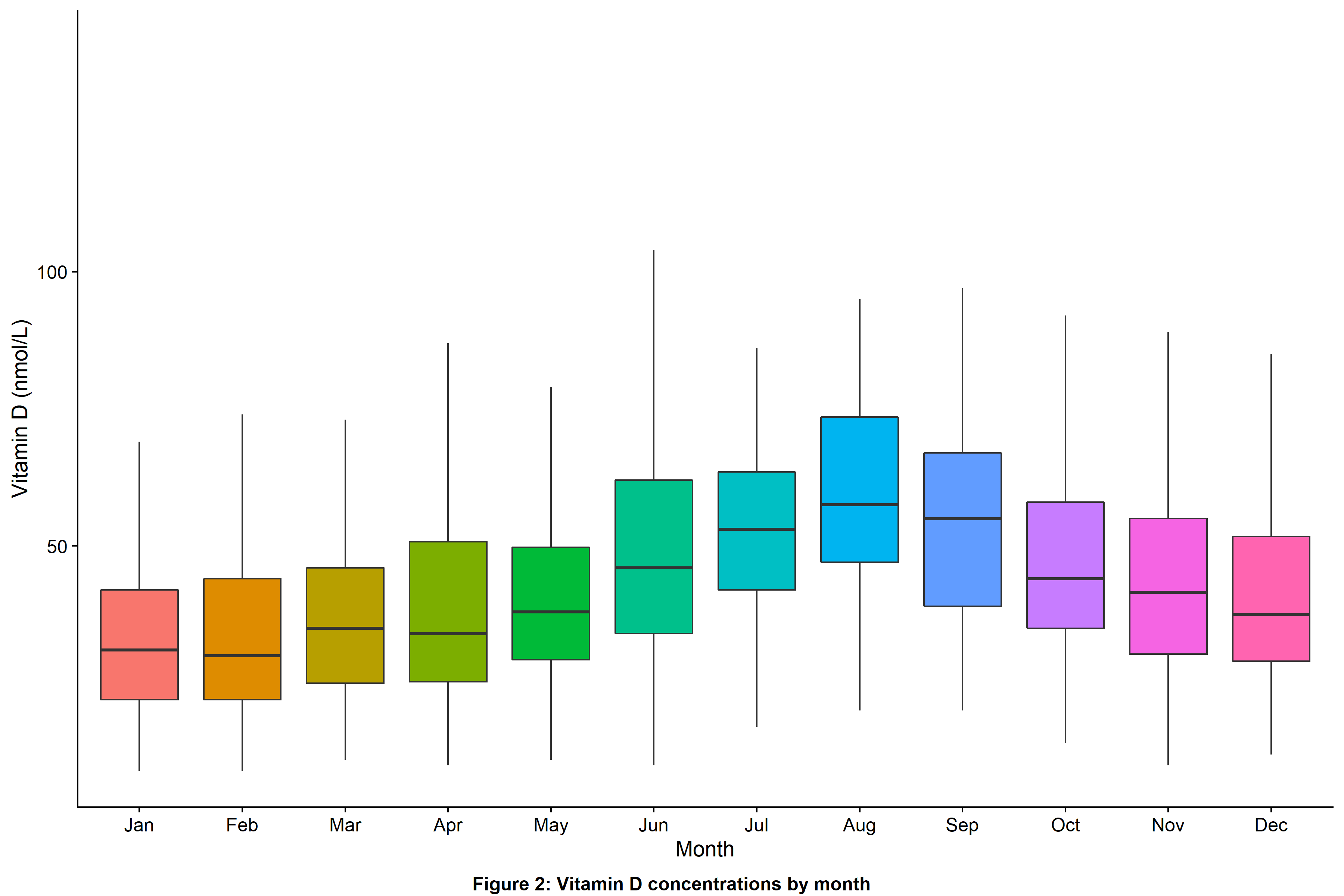
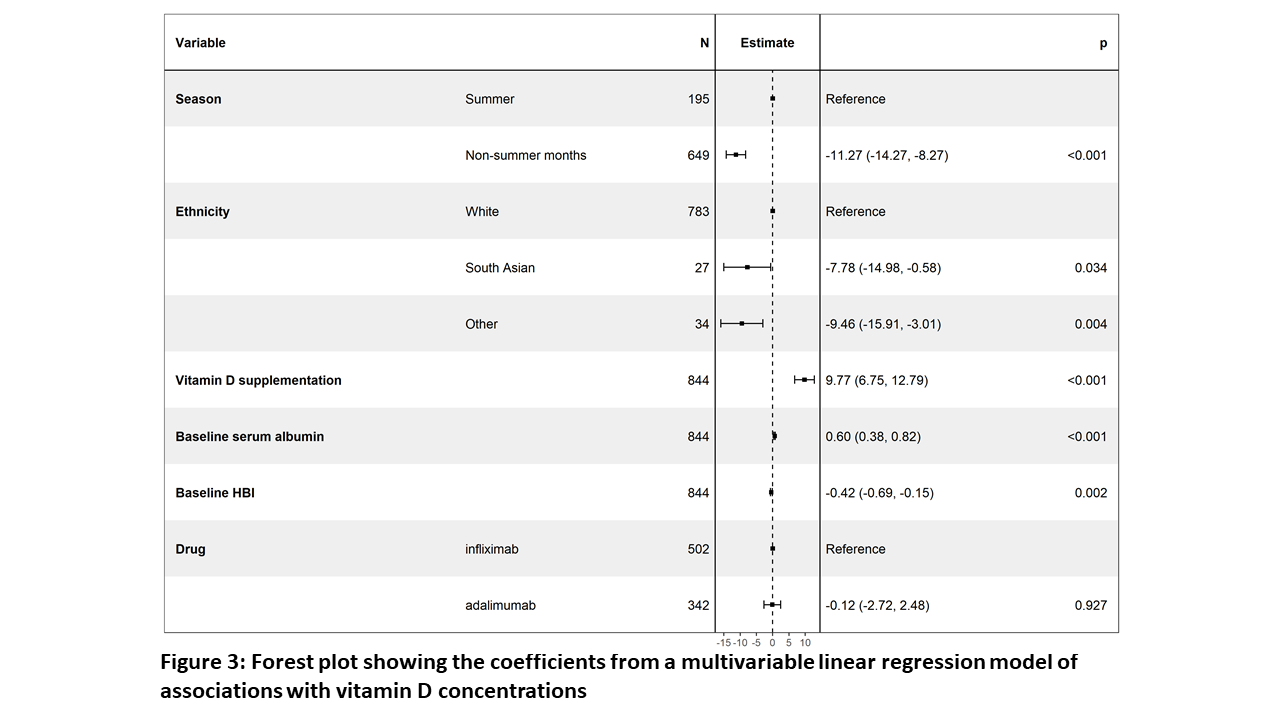
Multivariable linear regression analysis confirmed that baseline sampling during non-summer months (Figure 2), South Asian ethnicity, lower serum albumin concentrations, higher HBI and nontreatment with vitamin D supplements were independently associated with lower vitamin D concentrations (Figure 3).
Primary non-response at week 14 and non-remission at week 54 occurred in 19.3% (116/600; 95% CI 16.4 - 22.7%) and 58.8% (351/597; 95% CI 54.8- 62.7%) patients treated with infliximab and 25.3% (100/396; 95% CI 21.2- 29.8%) and 65.3% (246/377; 95% CI 60.3- 69.9%) of patients treated with adalimumab, respectively.
Pre-treatment vitamin D status did not predict response or remission status to anti-TNF therapy at week 14 (infliximab Ppnr = 0.87, adalimumab Ppnr = 0.18) or non-remission at week 54 (infliximab P = 0.12, adalimumab P = 0.59) (Figure 4).


Conclusion
Vitamin D deficiency is common in patients with active Crohn’s disease. Unlike previous studies, pre-treatment serum 25-hydroxyvitamin D concentration did not predict primary non-response to anti-TNF treatment at week 14 or non-remission at week 54.


